Nat Prod Sci.
2018 Mar;24(1):21-27. 10.20307/nps.2018.24.1.21.
The Amelioration Effect of the Ethanolic Extract of Cnidium officinale in Mice with Imiquimod-induced Psoriasis-like Skin Lesion
- Affiliations
-
- 1Hongcheon Institute of Medicinal Herb, Hongcheon 25142, Republic of Korea. wiscsyk@naver.com
- 2R&D Center, Radiant Ltd., Chuncheon 24398, Republic of Korea.
- 3Research and Development, Hankook Korus Pharm, Co., LTD., Chuncheon 24398, Republic of Korea.
- 4College of Pharmacy, Kangwon National University, Chuncheon 24341, Republic of Korea. heejyang@kangwon.ac.kr
- KMID: 2409605
- DOI: http://doi.org/10.20307/nps.2018.24.1.21
Abstract
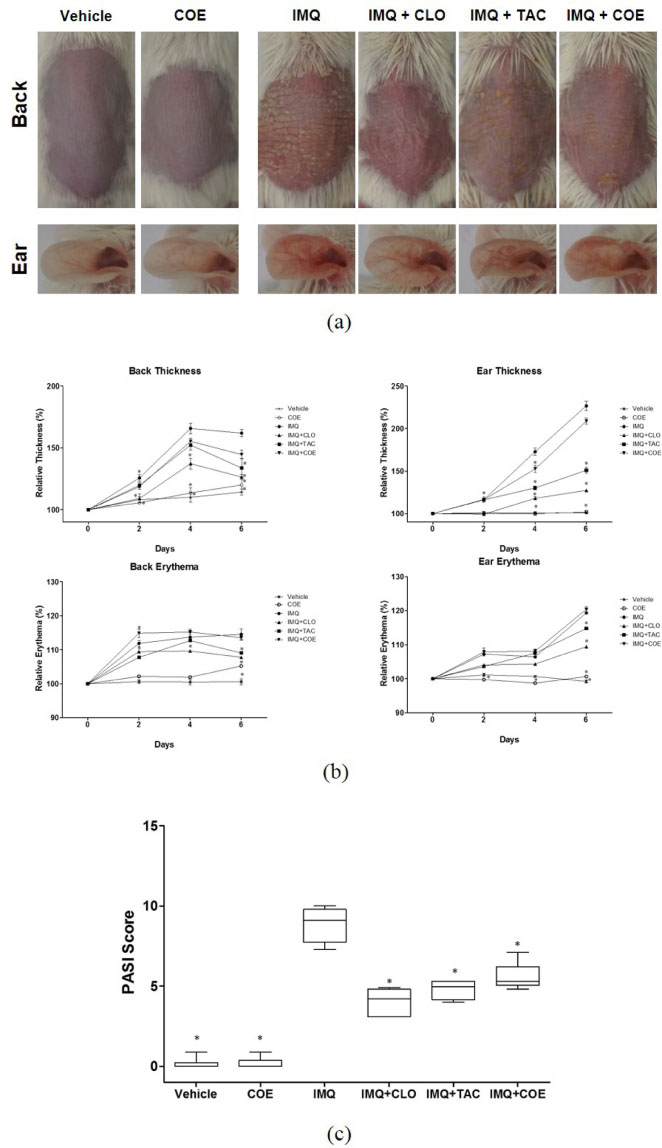
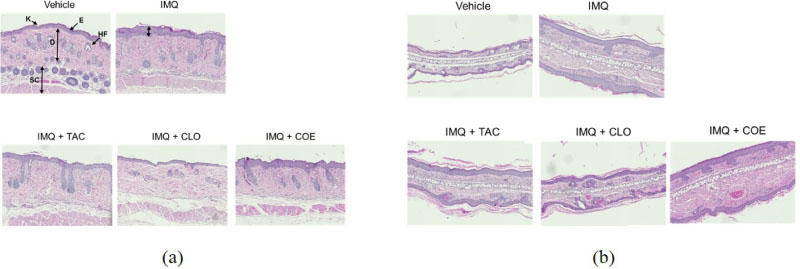
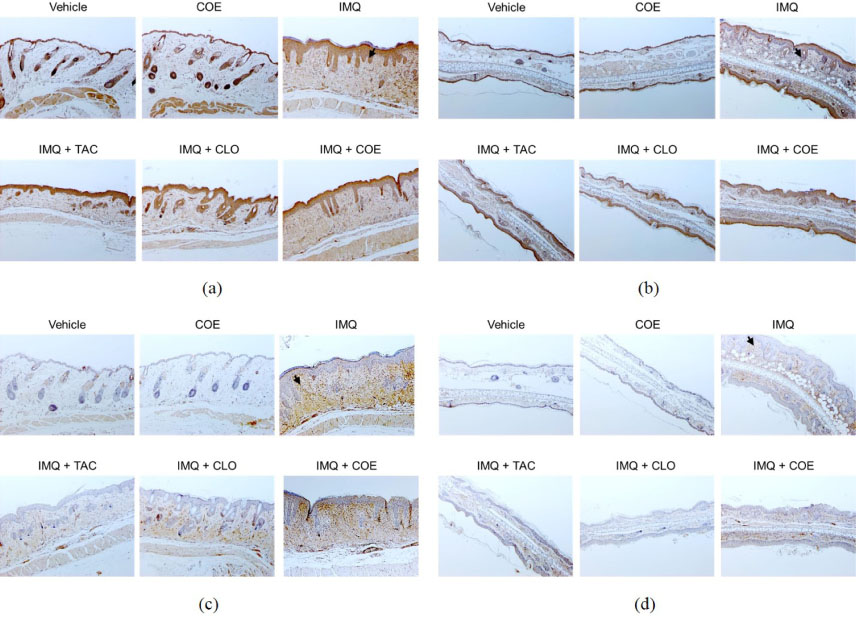
- Psoriasis is an auto-immune skin disease, which is characterized by the excessive generation of plaques on the skin with typically a long-lasting red, itchy and scaly symptoms. Imiquimod, which has been used for the treatment of external genital warts, actinic keratosis, and superficial basal cell carcinoma, induced of psoriasis-like skin disorders with skin erythema and thickness in mice. In the present study, we tried to find the bioactive herbal extract against imiquimod-induced psoriasis-like skin disorder in mice. During the searching of the herbal extract with anti-psoriatic effect, the ethanolic extract of Cnidium officinale ameliorated imiquimodinduced psoriasis-like skin disorder in mice. The morphological evaluation, H&E staining and Psoriasis Area and Severity Index (PASI) score showed that ear and back thickness, and erythema induced by imiquimod were significantly reversed after the treatment of the cream of the ethanolic extract of C. officinale. The overexpressed myeloperoxidase (MPO) and keratin 6A levels were decreased by the treatment of C. officinale cream. Also, IFN-γ, c-fos and IκB-α mRNA levels, which are related to the progression of psoriasis, were reduced by C. officinale cream. Thus, the ethanolic extract of C. officinale ameliorated psoriasis-like skin disorder induced by imiquimod and might be the therapeutic agent for psoriasis.
Keyword
MeSH Terms
Figure
Reference
-
1. Sakkas LI, Bogdanos DP. Autoimmun Rev. 2017; 16:10–15.2. Menter A, Gottlieb A, Feldman SR, Van Voorhees AS, Leonardi CL, Gordon KB, Lebwohl M, Koo JYM, Elmets CA, Korman NJ, Beutner KR, Bhushan R. J Am Acad Dermatol. 2008; 58:826–850.3. Zhang CS, Yu JJ, Parker S, Zhang AL, May B, Lu C, Xue CC. Int J Dermatol. 2014; 53:1305–1318.4. Deng S, May BH, Zhang AL, Lu C, Xue CCL. Br J Dermatol. 2013; 169:769–782.5. OuYang Q, Pan Y, Luo H, Xuan C, Liu J, Liu J. Int Immunopharmacol. 2016; 39:369–376.6. van der Fits L, Mourits S, Voerman JSA, Kant M, Boon L, Laman JD, Cornelissen F, Mus AM, Florencia E, Prens EP, Lubberts E. J Immunol. 2009; 182:5836–5845.7. Choi HS, Kim MSL, Sawamura M. Flavour Fragr J. 2002; 17:49–53.
Article8. Bae KE, Choi YW, Kim ST, Kim YK. Molecules. 2011; 16:8833–8847.9. Ramalingam M, Park YK. Pharmacogn Mag. 2010; 6:323–330.10. Wu Z, Uchi H, orino-Koga S, Nakamura-Satomura A, Kita K, Shi W, Furue M. Exp Dermatol. 2014; 23:260–265.11. Wu Z, Uchi H, Morino-Koga S, Shi W, Furue M. Exp Dermatol. 2015; 24:703–708.12. Vincent N, Ramya DD, Vedha HB. Dermatol Reports. 2014; 6:5451.13. Mommers JM, van Rossum MM, van Erp PEJ, Van de Kerkhof PCM. Dermatology. 2000; 201:15–20.14. Buates S, Matlashewski G. J Infect Dis. 1999; 179:1485–1494.
Article15. Palfreeman AC, McNamee KE, McCann FE. Drug Des Devel Ther. 2013; 7:201–210.16. Nestle FO, Kaplan DH, Barker J. N Engl J Med. 2009; 361:496–509.17. Hou R, Li J, Niu X, Liu R, Chang W, Zhao X, Wang Q, Li X, Yin G, Zhang K. J Dermatol Sci. 2017; 86:181–186.18. Han R, Rostami-Yazdi M, Gerdes S, Mrowietz U. Br J Clin Pharmacol. 2012; 74:424–436.19. Bello I, Shehu MW, Musa M, Asmawi MZ, Mahmud R. J Ethnopharmacol. 2016; 189:253–276.20. Choudhry SZ, Bhatia N, Ceilley R, Hougeir F, Lieberman R, Hamzavi I, Lim HW. J Drugs Dermatol. 2014; 13:148–153.21. Lee YM, Kim NH, Lee MR. Nat Prod Sci. 2015; 21:122–127.22. Belcaro G, Maquart FX, Scoccianti M, Dugall M, Hosoi M, Cesarone MR, Luzzi R, Cornelli U, Ledda A, Feragalli B. Panminerva Med. 2011; 53:105–118.23. Jeong JB, Ju SY, Park JH, Lee JR, Yun KW, Kwon ST, Lim JH, Chung GY, Jeong HJ. Cancer Epidemiol. 2009; 33:41–46.24. Jeong JB, Park JH, Lee HK, Ju SY, Hong SC, Lee JR, Chung GY, Lim JH, Jeong HJ. Food Chem Toxicol. 2009; 47:525–529.25. Cao LY, Soler DC, Debanne SM, Grozdev I, Rodriguez ME, Feig RL, Carman TL, Gilkeson RC, Orringer CE, Kern EF, McCormick TS, Cooper KD, Korman NJ. Am J Transl Res. 2013; 6:16–27.26. Freedberg IM, Tomic-Canic M, Komine M, Blumenberg M. J Invest Dermatol. 2001; 116:633–640.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Persicaria senticosa Ameliorates Imiquimod-induced Psoriasis-like Skin Lesions in Mice via Suppression of IL-6/STAT3 Expression and Proliferation of Keratinocytes
- Connective Tissue Growth Factor Neutralization Aggravates the Psoriasis Skin Lesion: The Analysis of Psoriasis Model Mice and Patients
- Immunohistochemical Study of Psoriasis-related Gene Expression in Imiquimod-induced Psoriasis-like Mouse Model
- Rhododendrin inhibits toll-like receptor-7-mediated psoriasis-like skin inflammation in mice
- Possible Role of Lysine Demethylase 2A in the Pathophysiology of Psoriasis