Perinatology.
2018 Mar;29(1):13-19. 10.14734/PN.2018.29.1.13.
Late and Insufficient Phosphorus Supplementation is Associated with Early Severe Hypophosphatemia in Extremely Low Birth Weight Infants with Early Amino Acid Administration
- Affiliations
-
- 1Department of Pediatrics, Ajou University School of Medicine, Suwon, Korea. neopedlee@gmail.com
- 2Department of Radiation Oncology, Ajou University School of Medicine, Suwon, Korea.
- 3Department of Biomedical Informatics, Ajou University School of Medicine, Suwon, Korea.
- KMID: 2409107
- DOI: http://doi.org/10.14734/PN.2018.29.1.13
Abstract
OBJECTIVE
To investigate the incidence of early severe hypophosphatemia, and examine the associated clinical factors and outcomes, in extremely low birth weight infants (ELBWI) who received early amino acid administration.
METHODS
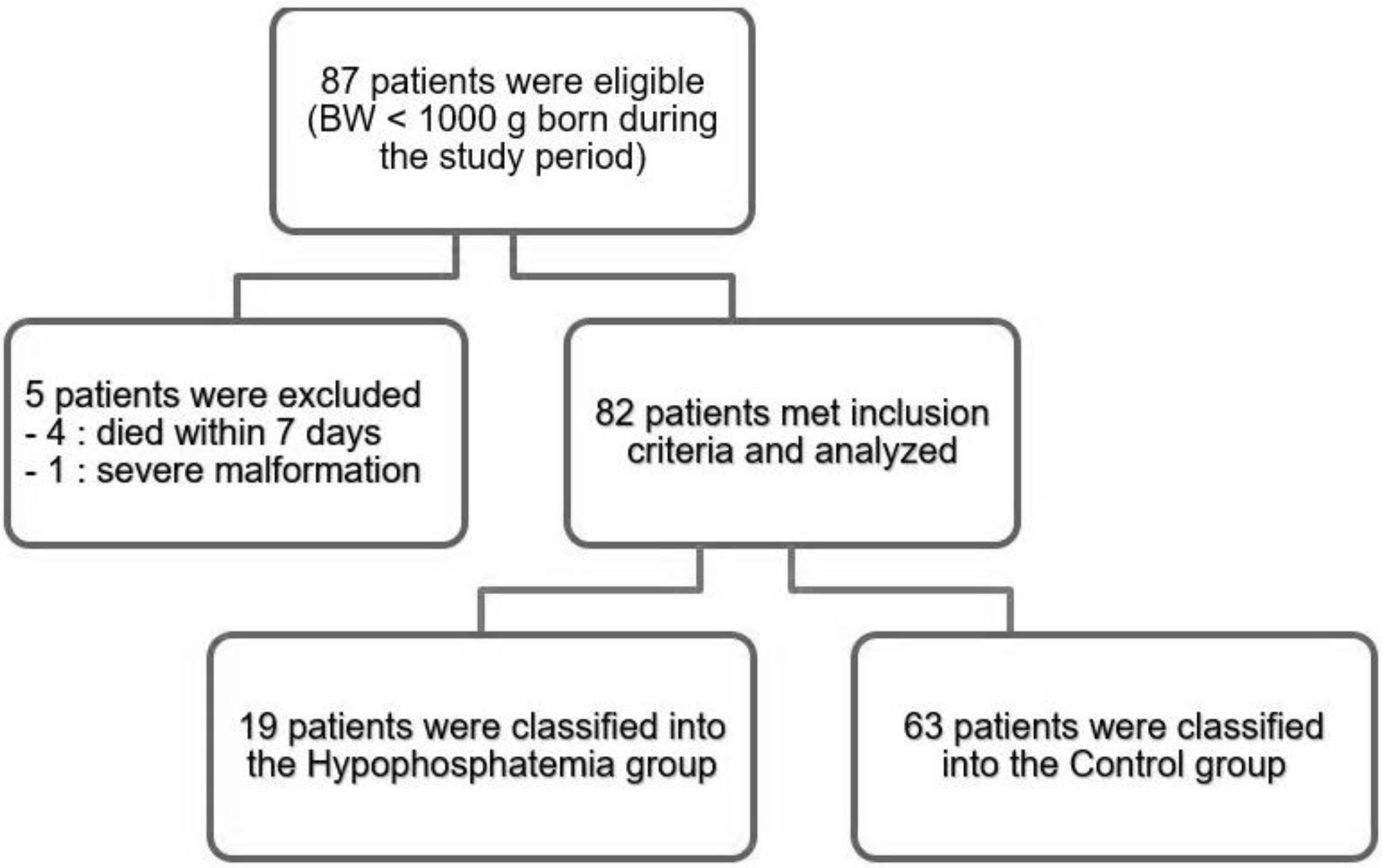
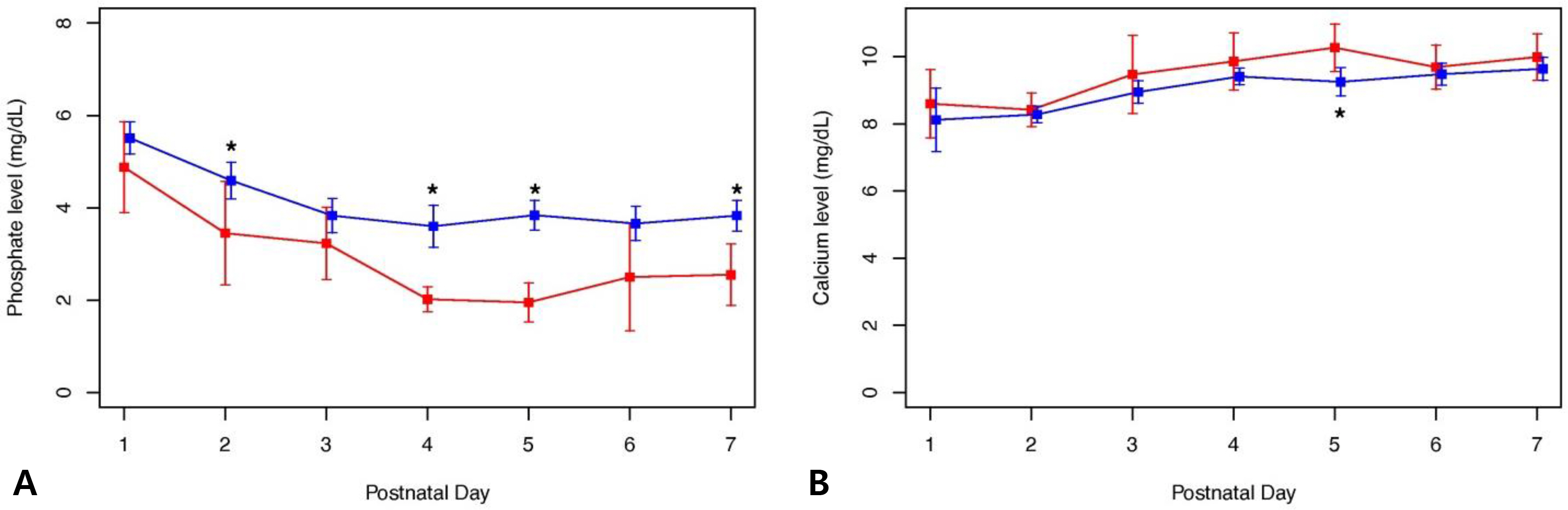
Medical records of 82 ELBWI were retrospectively reviewed. Severe hypophosphatemia was defined as a serum phosphate level < 2 mg/dL during the first week after birth.
RESULTS
Nineteen ELBWI (23.2%) experienced severe hypophosphatemia. The supplementation of phosphorus was started significantly later in the hypophosphatemia group compared to that in the control group (P=0.036). Small for gestational age infants (SGAI) (P=0.006) and bronchopulmonary dysplasia (P=0.016) were more prevalent in the hypophosphatemia group compared to that in the control group.
CONCLUSION
Early severe hypophosphatemia is common in ELBWI. Late and insufficient supplementation of phosphorus and SGAI were associated with severe hypophosphatemia.
MeSH Terms
Figure
Reference
-
1). Valentine CJ., Fernandez S., Rogers LK., Gulati P., Hayes J., Lore P, et al. Early amino-acid administration improves preterm infant weight. J Perinatol. 2009. 29:428–32.
Article2). Koletzko B., Goulet O., Hunt J., Krohn K., Shamir R. Guidelines on paediatric parenteral nutrition of the european society of paediatric gastroenterology, hepatology and nutrition (ESPGHAN) and the European Society for Clinical Nutrition and Metabolism (ESPEN), supported by the European Society of Paediatric Research (ESPR). J Pediatr Gastroenterol Nutr. 2005. 41(Suppl 2):S1–87.3). Thureen PJ., Melara D., Fennessey PV., Hay WW Jr. Effect of low versus high intravenous amino acid intake on very low birth weight infants in the early neonatal period. Pediatr Res. 2003. 53:24–32.
Article4). Brener Dik PH., Galletti MF., Fernández Jonusas SA., Alonso G., Mariani GL., Fustiñana CA. Early hypophosphatemia in preterm infants receiving aggressive parenteral nutrition. J Perinatol. 2015. 35:712–5.
Article5). Skipper A. Refeeding syndrome or refeeding hypophosphatemia: a systematic review of cases. Nutr Clin Pract. 2012. 27:34–40.6). Ross JR., Finch C., Ebeling M., Taylor SN. Refeeding syndrome in very-low-birth-weight intrauterine growth-restricted neonates. J Perinatol. 2013. 33:717–20.
Article7). Ichikawa G., Watabe Y., Suzumura H., Sairenchi T., Muto T., Arisaka O. Hypophosphatemia in small for gestational age extremely low birth weight infants receiving parenteral nutrition in the first week after birth. J Pediatr Endocrinol Metab. 2012. 25:317–21.
Article8). Boubred F., Herlenius E., Bartocci M., Jonsson B., Vanpee M. Extremely preterm infants who are small for gestational age have a high risk of early hypophosphatemia and hypokalemia. Acta Paediatr. 2015. 104:1077–83.
Article9). Moe K., Beck-Nielsen SS., Lando A., Greisen G., Zachariassen G. Administering different levels of parenteral phosphate and amino acids did not influence growth in extremely preterm infants. Acta Paediatr. 2015. 104:894–9.10). Jobe AH., Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001. 163:1723–9.
Article11). Neu J. Necrotizing enterocolitis: the search for a unifying pathogenic theory leading to prevention. Pediatr Clin North Am. 1996. 43:409–32.12). Papile LA., Burstein J., Burstein R., Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978. 92:529–34.
Article13). International Committee for the Classification of Retinopathy of Prematurity. The international classification of retinopathy of prematurity revisited. Arch Ophthalmol. 2005. 123:991–9.14). Fenton TR. A new growth chart for preterm babies: Babson and Benda's chart updated with recent data and a new format. BMC Pediatr. 2003. 3:13.
Article15). Team RC. R: a language and environment for the statistical computing. [accessed on 16 Oct 2017]. Available at. https://cran.r-project.org/mirrors.html.16). Bonsante F., Iacobelli S., Latorre G., Rigo J., De Felice C., Robillard PY, et al. Initial amino acid intake influences phosphorus and calcium homeostasis in preterm infants—it is time to change the composition of the early parenteral nutrition. PLoS One. 2013. 8:e72880.17). Mizumoto H., Mikami M., Oda H., Hata D. Refeeding syndrome in a small-for-dates micro-preemie receiving early parenteral nutrition. Pediatr Int. 2012. 54:715–7.
Article18). Marik PE., Bedigian MK. Refeeding hypophosphatemia in critically ill patients in an intensive care unit. A prospective study. Arch Surg. 1996. 131:1043–7.19). Dreyfus L., Fischer Fumeaux CJ., Remontet L., Essomo Megnier Mbo Owono MC., Laborie S., Maucort-Boulch D, et al. Low phosphatemia in extremely low birth weight neonates: a risk factor for hyperglycemia? Clin Nutr. 2016. 35:1059–65.
Article20). Zhao Y., Li Z., Shi Y., Cao G., Meng F., Zhu W, et al. Effect of hypophosphatemia on the withdrawal of mechanical ventilation in patients with acute exacerbations of chronic obstructive pulmonary disease. Biomed Rep. 2016. 4:413–6.
Article21). Kilic O., Demirkol D., Ucsel R., Citak A., Karabocuoglu M. Hypophosphatemia and its clinical implications in critically ill children: a retrospective study. J Crit Care. 2012. 27:474–9.
Article22). Gustavsson CG., Eriksson L. Acute respiratory failure in anorexia nervosa with hypophosphataemia. J Intern Med. 1989. 225:63–4.
Article23). Hasselstrøm L., Wimberley PD., Nielsen VG. Hypophosphatemia and acute respiratory failure in a diabetic patient. Intensive Care Med. 1986. 12:429–31.
Article24). Liu PY., Jeng CY. Severe hypophosphatemia in a patient with diabetic ketoacidosis and acute respiratory failure. J Chin Med Assoc. 2004. 67:355–9.25). Aubier M., Murciano D., Lecocguic Y., Viires N., Jacquens Y., Squara P, et al. Effect of hypophosphatemia on diaphragmatic contractility in patients with acute respiratory failure. N Engl J Med. 1985. 313:420–4.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Early Phosphorus Intake on Respiratory Distress in Extremely Low-Birth-Weight Infants
- Nutritional strategy of early amino acid administration in very low birth weight infants
- Blood Urea Nitrogen Concentration and Aggressive Parenteral Amino Acid Administration in Extremely Low Birth Weight Infants during the First Week
- Effects of Early Parenteral Nutrition for Extremely Low Birth Weight Infants
- Tolerability and Effect of Early High-Dose Amino Acid Administration in Extremely Low Birth Weight Infants