Allergy Asthma Immunol Res.
2018 May;10(3):236-243. 10.4168/aair.2018.10.3.236.
Analysis of Peripheral B Cell Subsets in Patients With Allergic Rhinitis
- Affiliations
-
- 1Department of Immunology, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China.
- 2Department of Otolaryngology, Zhongnan Hospital of Wuhan University, Wuhan, China. zhouxuhong@126.com, allergyli@163.com
- 3Department of Otolaryngology, Head and Neck Surgery, Affiliated Eye, Ear, Nose and Throat Hospital, Fudan University, Shanghai, China.
- 4State Key Laboratory of Respiratory Disease, Department of Otolaryngology, Head and Neck Surgery, The First Affiliated Hospital of Guangzhou Medical University, Guangzhou, China.
- KMID: 2409058
- DOI: http://doi.org/10.4168/aair.2018.10.3.236
Abstract
- PURPOSE
Recent evidence suggests that B cells can both promote and inhibit the development and progression of allergic disease. However, the characteristics of B cell subsets in patients with allergic rhinitis (AR) have not been well documented. This study aimed to analyze the characteristics of B cell subsets in the peripheral blood of AR patients.
METHODS
Forty-seven AR patients and 54 healthy controls were enrolled in this study, and the B cell subsets in peripheral blood of all subjects were analyzed by flow cytometry. Moreover, the serum total immunoglobulin E (IgE) and IgE concentrations secreted into the cultured peripheral blood mononuclear cells (PBMCs) were measured by using enzyme-linked immunosorbent assay.
RESULTS
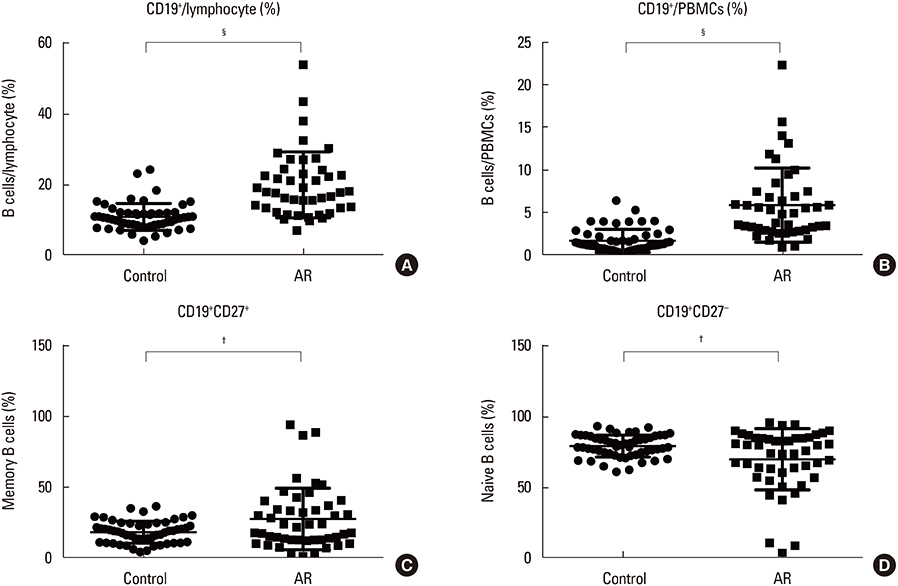
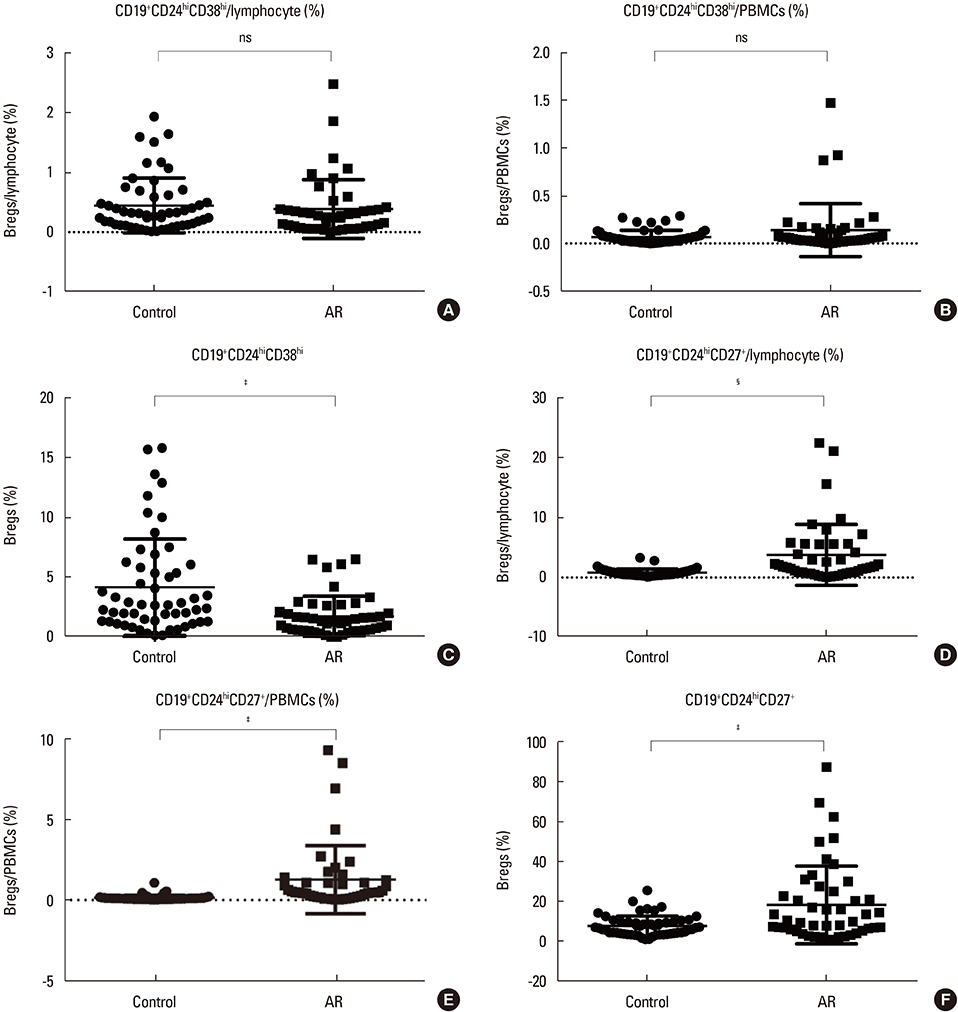
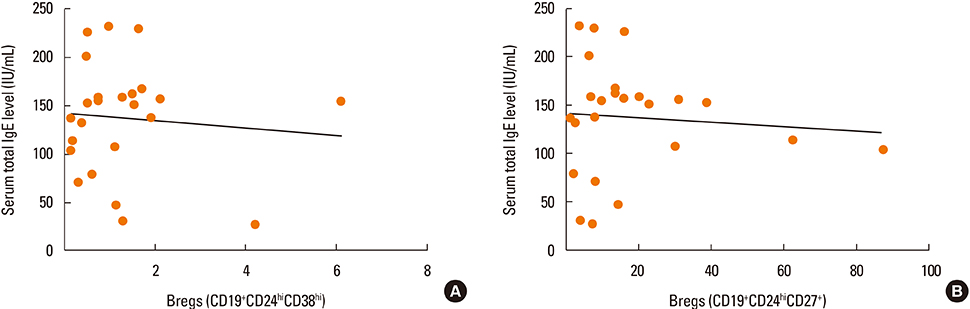
We found the peripheral blood of AR patients contained higher percentages of memory B cells, plasma cells, and CD19+CD24hiCD27+ regulatory B cells (Bregs) than those of age-matched healthy controls (P < 0.05), while the percentages of naïve B cells and CD19+CD24hiCD38hi Bregs were significantly lower in AR patients than in healthy individuals (P < 0.05). In addition, the serum total IgE and IgE concentrations secreted into the cultured PBMCs were elevated in AR patients than in the healthy controls (P < 0.05).
CONCLUSIONS
Our findings indicate that AR patients were characterized by increase in terminally differentiated memory B cells or plasma cells and decreases in CD19+CD24hiCD38hi Breg cells in the peripheral blood.
MeSH Terms
Figure
Reference
-
1. Greiner AN, Hellings PW, Rotiroti G, Scadding GK. Allergic rhinitis. Lancet. 2011; 378:2112–2122.
Article2. Shaaban R, Zureik M, Soussan D, Neukirch C, Heinrich J, Sunyer J, et al. Rhinitis and onset of asthma: a longitudinal population-based study. Lancet. 2008; 372:1049–1057.
Article3. Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2) LEN and AllerGen). Allergy. 2008; 63:Suppl 86. 8–160.4. Yoo KH, Ahn HR, Park JK, Kim JW, Nam GH, Hong SK, et al. Burden of respiratory disease in Korea: an observational study on allergic rhinitis, asthma, COPD, and rhinosinusitis. Allergy Asthma Immunol Res. 2016; 8:527–534.
Article5. Wu LC, Zarrin AA. The production and regulation of IgE by the immune system. Nat Rev Immunol. 2014; 14:247–259.
Article6. Eifan AO, Durham SR. Pathogenesis of rhinitis. Clin Exp Allergy. 2016; 46:1139–1151.
Article7. Jutel M, Agache I, Bonini S, Burks AW, Calderon M, Canonica W, et al. International Consensus on Allergen Immunotherapy II: mechanisms, standardization, and pharmacoeconomics. J Allergy Clin Immunol. 2016; 137:358–368.
Article8. Rhee CS. Current specific immunotherapy for allergic rhinitis: perspectives from otorhinolaryngologists. Allergy Asthma Immunol Res. 2014; 6:273–275.
Article9. Celiksoy MH, Sancak R, Yildiran A. The role of active B cells in allergen immunotherapy. Allergol Immunopathol (Madr). 2017; 45:439–444.
Article10. Kamekura R, Shigehara K, Miyajima S, Jitsukawa S, Kawata K, Yamashita K, et al. Alteration of circulating type 2 follicular helper T cells and regulatory B cells underlies the comorbid association of allergic rhinitis with bronchial asthma. Clin Immunol. 2015; 158:204–211.
Article11. Xiao L, Wei Y, Zhang YN, Luo X, Yang BY, Yu SF, et al. Increased IL-21 expression in chronic rhinosinusitis with nasal polyps. Clin Exp Allergy. 2015; 45:404–413.12. Noh J, Noh G. Allergen-specific responses of CD19(high) and CD19 (low) B cells in Non-IgE-mediated food allergy of late eczematous reactions in atopic dermatitis: presence of IL-17- and IL-32-producing regulatory B cells (Br17 & Br32). Inflamm Allergy Drug Targets. 2012; 11:320–329.13. Klein U, Rajewsky K, Kuppers R. Human immunoglobulin (Ig)M+ IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J Exp Med. 1998; 188:1679–1689.14. Lee KM, Stott RT, Zhao G, SooHoo J, Xiong W, Lian MM, et al. TGF-β-producing regulatory B cells induce regulatory T cells and promote transplantation tolerance. Eur J Immunol. 2014; 44:1728–1736.
Article15. Mizoguchi A, Bhan AK. A case for regulatory B cells. J Immunol. 2006; 176:705–710.
Article16. Mauri C, Menon M. Human regulatory B cells in health and disease: therapeutic potential. J Clin Invest. 2017; 127:772–779.
Article17. Palomares O, Akdis M, Martín-Fontecha M, Akdis CA. Mechanisms of immune regulation in allergic diseases: the role of regulatory T and B cells. Immunol Rev. 2017; 278:219–236.
Article18. Blair PA, Norena LY, Flores-Borja F, Rawlings DJ, Isenberg DA, Ehrenstein MR, et al. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity. 2010; 32:129–140.
Article19. Sumimoto K, Uchida K, Kusuda T, Mitsuyama T, Sakaguchi Y, Fukui T, et al. The role of CD19+ CD24high CD38high and CD19+ CD24high CD27+ regulatory B cells in patients with type 1 autoimmune pancreatitis. Pancreatology. 2014; 14:193–200.20. Iwata Y, Matsushita T, Horikawa M, Dilillo DJ, Yanaba K, Venturi GM, et al. Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood. 2011; 117:530–541.
Article21. Jin L, Weiqian C, Lihuan Y. Peripheral CD24hi CD27+ CD19+ B cells subset as a potential biomarker in naive systemic lupus erythematosus. Int J Rheum Dis. 2013; 16:698–708.22. Quan C, ZhangBao J, Lu J, Zhao C, Cai T, Wang B, et al. The immune balance between memory and regulatory B cells in NMO and the changes of the balance after methylprednisolone or rituximab therapy. J Neuroimmunol. 2015; 282:45–53.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diagnosis of Allergic Rhinitis
- Allergic Rhinitis Mouse Model
- Allergic Rhinitis and Sleep-disordered Breathing
- The Role of Aviation Medical Examiners in the Diagnosis, Treatment and Aeromedical Assessment of Patients with Allergic Rhinitis
- Dermographism ( IV ): The Prevalence in Atopic Dermatitis and Allergic Rhinitis