Korean J Ophthalmol.
2018 Apr;32(2):116-125. 10.3341/kjo.2017.0041.
Prognostic Factors for Functional and Anatomic Outcomes in Patients with Diabetic Macular Edema Treated with Dexamethasone Implant
- Affiliations
-
- 1Department of Ophthalmology, Konkuk University Medical Center, Konkuk University School of Medicine, Seoul, Korea. eyekim@kuh.ac.kr
- KMID: 2408951
- DOI: http://doi.org/10.3341/kjo.2017.0041
Abstract
- PURPOSE
To investigate the prognostic factors of visual and anatomic outcomes in patients with diabetic macular edema (DME) treated with intravitreal injection of dexamethasone implant.
METHODS
We retrospectively studied 32 eyes of 31 patients with DME for best-corrected visual acuity (BCVA), central macular thickness, and height and width of both intraretinal fluid (IRF) and subretinal fluid. Logistic regression analysis was used to examine correlations between the baseline characteristics and outcomes at 3 and 6 months.
RESULTS
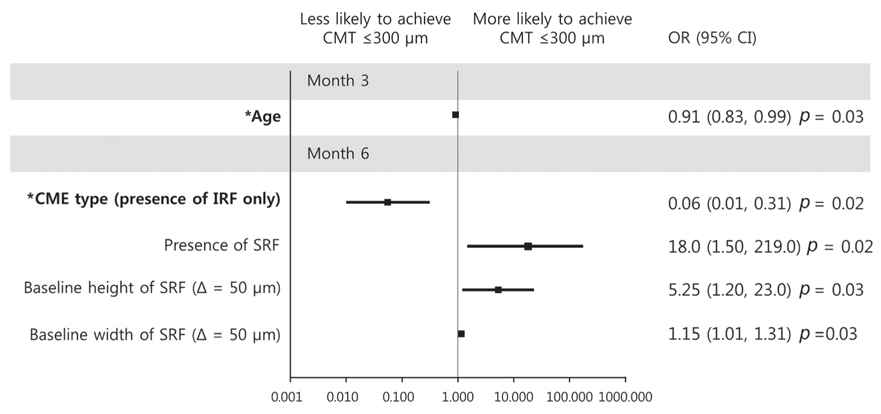
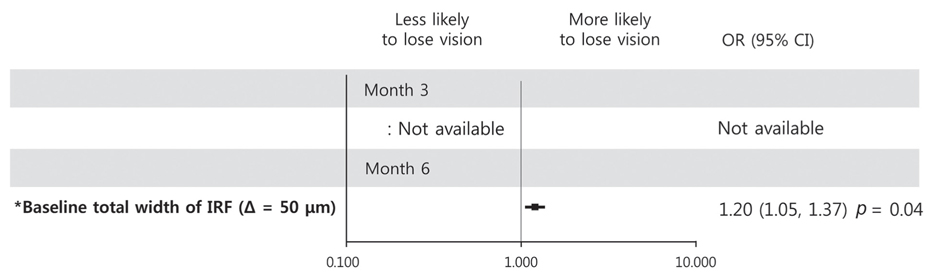
Baseline predictor of BCVA ≥20 / 40 at month 3 was short height of baseline IRF (p = 0.02), while good baseline BCVA was a predictor for month 6 (p = 0.01). Predictors of improvement in logarithm of minimum angle of resolution BCVA 0.2 at month 3 were the absence of baseline IRF and poor baseline BCVA (p = 0.02 and p = 0.009, respectively), while poor baseline BCVA was the sole predictor at month 6 (p = 0.01). Predictor of central macular thickness ≤300 µm at month 3 was younger age (p = 0.03), while the absence of IRF was the predictor for BCVA improvement at month 6 (p = 0.02). BCVA ≤20 / 100 at month 3 was predicted by poor baseline BCVA (p = 0.01), and increased width of total IRF was the predictor at month 6 (p = 0.02). Predictor of loss of logarithm of minimum angle of resolution BCVA 0.2 at month 6 was increased width of total IRF at baseline (p = 0.04). Additional injection within 6 months was negatively associated with the presence of baseline DME (p = 0.03).
CONCLUSIONS
The visual and anatomical outcome of DME treatment with dexamethasone implant can be predicted by baseline visual acuity and IRF morphology.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
One-year Outcome of Intravitreal Dexamethasone Implant for Diabetic Macular Edema Patients
No Hae Park, Hyun Duck Kwak, Chang Ki Yoon, Ji Eun Lee, Min Sagong, Sang Joon Lee, Joo Eun Lee, Kun Hyung Kim, Hyun Woong Kim
J Korean Ophthalmol Soc. 2019;60(2):135-143. doi: 10.3341/jkos.2019.60.2.135.
Reference
-
1. Klein R, Klein BE, Moss SE, et al. The Wisconsin epidemiologic study of diabetic retinopathy. IV. Diabetic macular edema. Ophthalmology. 1984; 91:1464–1474.2. Bhagat N, Grigorian RA, Tutela A, Zarbin MA. Diabetic macular edema: pathogenesis and treatment. Surv Ophthalmol. 2009; 54:1–32.
Article3. Dugel PU, Bandello F, Loewenstein A. Dexamethasone intravitreal implant in the treatment of diabetic macular edema. Clin Ophthalmol. 2015; 9:1321–1335.
Article4. Zhioua I, Semoun O, Lalloum F, Souied EH. Intravitreal dexamethasone implant in patients with ranibizumab persistent diabetic macular edema. Retina. 2015; 35:1429–1435.
Article5. Moon BG, Lee JY, Yu HG, et al. Efficacy and safety of a dexamethasone implant in patients with diabetic macular edema at tertiary centers in Korea. J Ophthalmol. 2016; 2016:9810270.
Article6. Otani T, Kishi S, Maruyama Y. Patterns of diabetic macular edema with optical coherence tomography. Am J Ophthalmol. 1999; 127:688–693.
Article7. Kim BY, Smith SD, Kaiser PK. Optical coherence tomographic patterns of diabetic macular edema. Am J Ophthalmol. 2006; 142:405–412.
Article8. Seo KH, Yu SY, Kim M, Kwak HW. Visual and morphologic outcomes of intravitreal ranibizumab for diabetic macular edema based on optical coherence tomography patterns. Retina. 2016; 36:588–595.
Article9. Mori Y, Murakami T, Suzuma K, et al. Relation between macular morphology and treatment frequency during twelve months with ranibizumab for diabetic macular edema. PLoS One. 2017; 12:e0175809.
Article10. Giocanti-Auregan A, Hrarat L, Qu LM, et al. Functional and anatomical outcomes in patients with serous retinal detachment in diabetic macular edema treated with ranibizumab. Invest Ophthalmol Vis Sci. 2017; 58:797–800.11. Pelosini L, Hull CC, Boyce JF, et al. Optical coherence tomography may be used to predict visual acuity in patients with macular edema. Invest Ophthalmol Vis Sci. 2011; 52:2741–2748.
Article12. Shimura M, Nakazawa T, Yasuda K, et al. Comparative therapy evaluation of intravitreal bevacizumab and triamcinolone acetonide on persistent diffuse diabetic macular edema. Am J Ophthalmol. 2008; 145:854–861.
Article13. Shah SU, Harless A, Bleau L, Maturi RK. Prospective randomized subject-masked study of intravitreal bevacizumab monotherapy versus dexamethasone implant monotherapy in the treatment of persistent diabetic macular edema. Retina. 2016; 36:1986–1996.
Article14. Sophie R, Lu N, Campochiaro PA. Predictors of functional and anatomic outcomes in patients with diabetic macular edema treated with ranibizumab. Ophthalmology. 2015; 122:1395–1401.
Article15. Ferris FL 3rd, Patz A. Macular edema: a complication of diabetic retinopathy. Surv Ophthalmol. 1984; 28:Suppl. 452–461.
Article16. Bressler SB, Qin H, Beck RW, et al. Factors associated with changes in visual acuity and central subfield thickness at 1 year after treatment for diabetic macular edema with ranibizumab. Arch Ophthalmol. 2012; 130:1153–1161.
Article17. Yanoff M, Fine BS, Brucker AJ, Eagle RC Jr. Pathology of human cystoid macular edema. Surv Ophthalmol. 1984; 28:Suppl. 505–511.
Article18. Bringmann A, Reichenbach A, Wiedemann P. Pathomechanisms of cystoid macular edema. Ophthalmic Res. 2004; 36:241–249.
Article19. Spaide RF. Retinal vascular cystoid macular edema: review and new theory. Retina. 2016; 36:1823–1842.20. Romero-Aroca P, Baget-Bernaldiz M, Pareja-Rios A, et al. Diabetic macular edema pathophysiology: vasogenic versus inflammatory. J Diabetes Res. 2016; 2016:2156273.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Inadvertent Intralenticular Dexamethasone Implant for Diabetic Macular Edema Unresponsive to Bevacizumab
- Short-Term Results of Dexamethasone Intravitreal Implant in Patients with Refractory Diabetic Macular Edema
- Comparison of Optical Coherence Tomography Biomarkers between Bevacizumab Good Responders and Nonresponders Who were Switched to Dexamethasone Implant in Diabetic Macular Edema
- A Case of Repeated Intravitreal Dexamethasone Implantation for Treatment of Macular Edema after Scleral Fixation of Intraocular Lens
- A Multimodal Approach to Diabetic Macular Edema