Investig Magn Reson Imaging.
2018 Mar;22(1):65-70. 10.13104/imri.2018.22.1.65.
Radiofrequency Coil Design for in vivo Sodium Magnetic Resonance Imaging of Mouse Kidney at 9.4T
- Affiliations
-
- 1Asan Institute for Life Sciences, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. dcwoo@amc.seoul.kr
- 2Department of Convergence Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 3Department of Biomedical Engineering, Research Institute of Biomedical Engineering, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- KMID: 2408820
- DOI: http://doi.org/10.13104/imri.2018.22.1.65
Abstract
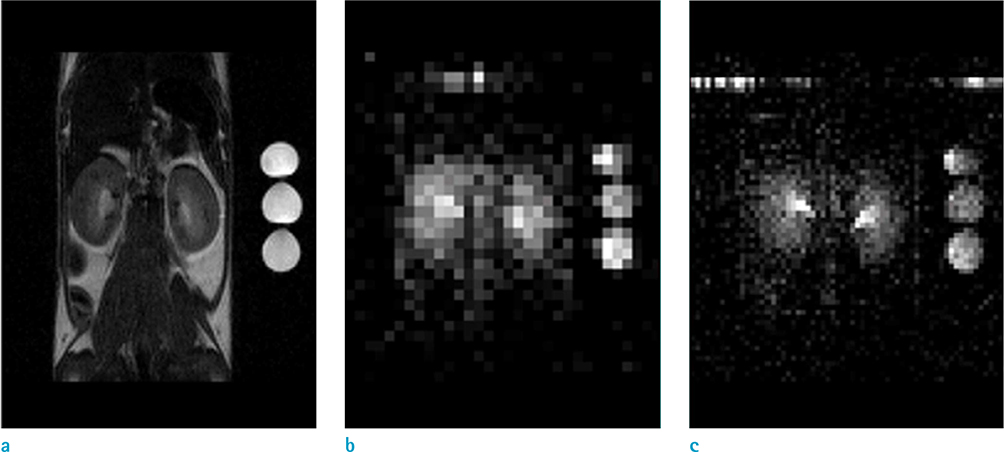
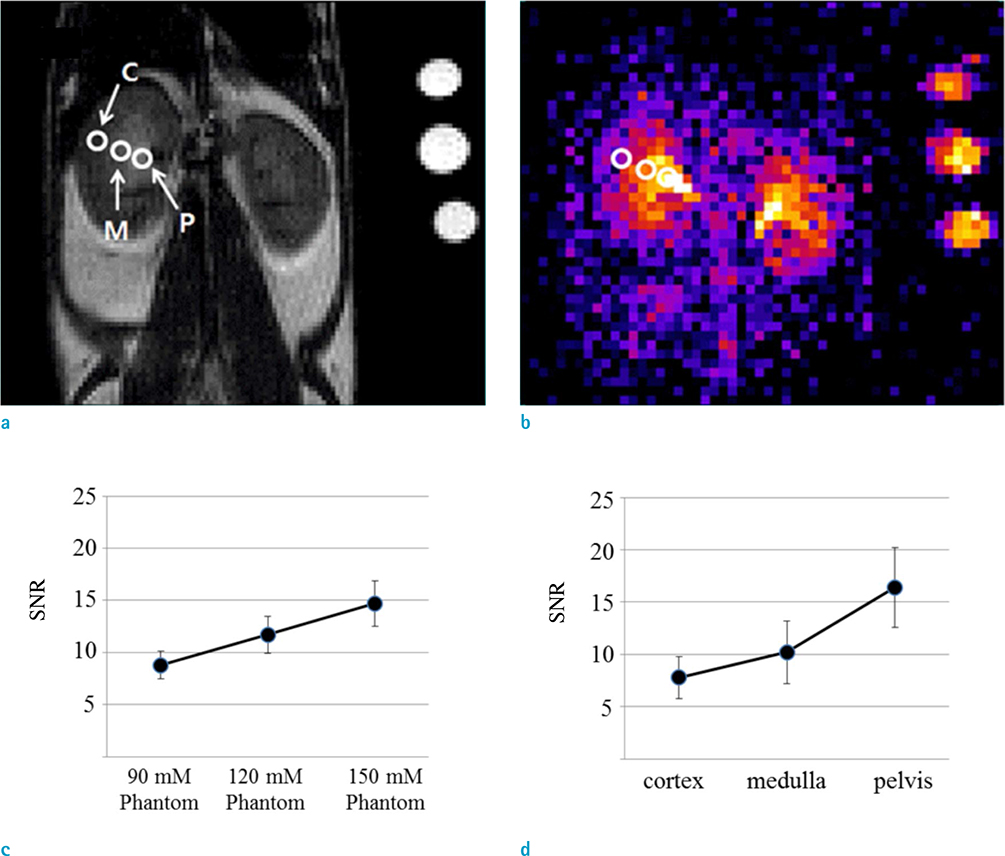
- The objective of this study was to describe a radiofrequency (RF) coil design for in vivo sodium magnetic resonance imaging (MRI) for use in small animals. Accumulating evidence has indicated the importance and potential of sodium imaging with improved magnet strength (> 7T), faster gradient, better hardware, multi-nucleus imaging methods, and optimal coil design for patient and animal studies. Thus, we developed a saddle-shaped sodium volume coil with a diameter/length of 30/30 mm. To evaluate the efficiency of this coil, bench-level measurement was performed. Unloaded Q value, loaded Q value, and ratio of these two values were estimated to be 352.8, 211.18, and 1.67, respectively. Thereafter, in vivo acquisition of sodium images was performed using normal mice (12 weeks old; n = 5) with a two-dimensional gradient echo sequence and minimized echo time to increase spatial resolution of images. Sodium signal-to-noise ratio in mouse kidneys (renal cortex, medulla, and pelvis) was measured. We successfully acquired sodium MR images of the mouse kidney with high spatial resolution (approximately 0.625 mm) through a combination of sodium-proton coils.
Keyword
Figure
Reference
-
1. Maiti AK, Islam MT, Satou R, Majid DS. Enhancement in cellular Na+K+ATPase activity by low doses of peroxynitrite in mouse renal tissue and in cultured HK2 cells. Physiol Rep. 2016; 4.
Article2. Wetterling F, Tabbert M, Junge S, Gallagher L, Macrae IM, Fagan AJ. A double-tuned (1)H/(23)Na dual resonator system for tissue sodium concentration measurements in the rat brain via Na-MRI. Phys Med Biol. 2010; 55:7681–7695.3. Haneder S, Juras V, Michaely HJ, et al. In vivo sodium (23Na) imaging of the human kidneys at 7 T: preliminary results. Eur Radiol. 2014; 24:494–501.4. Wetterling F, Hogler M, Molkenthin U, et al. The design of a double-tuned two-port surface resonator and its application to in vivo hydrogen- and sodium-MRI. J Magn Reson. 2012; 217:10–18.
Article5. Brown R, Lakshmanan K, Madelin G, et al. A flexible nested sodium and proton coil array with wideband matching for knee cartilage MRI at 3T. Magn Reson Med. 2016; 76:1325–1334.
Article6. Lykowsky G, Carinci F, During M, Weber D, Jakob PM, Haddad D. Optimization and comparison of two practical dual-tuned birdcage configurations for quantitative assessment of articular cartilage with sodium magnetic resonance imaging. Quant Imaging Med Surg. 2015; 5:799–805.7. Hattori K, Ikemoto Y, Takao W, et al. Development of MRI phantom equivalent to human tissues for 3.0-T MRI. Med Phys. 2013; 40:032303.
Article8. Constantinides CD, Gillen JS, Boada FE, Pomper MG, Bottomley PA. Human skeletal muscle: sodium MR imaging and quantification-potential applications in exercise and disease. Radiology. 2000; 216:559–568.
Article9. Moon CH, Furlan A, Kim JH, Zhao T, Shapiro R, Bae KT. Quantitative sodium MR imaging of native versus transplanted kidneys using a dual-tuned proton/sodium (1H/23Na) coil: initial experience. Eur Radiol. 2014; 24:1320–1326.
Article10. Roemer PB, Edelstein WA, Hayes CE, Souza SP, Mueller OM. The NMR phased array. Magn Reson Med. 1990; 16:192–225.
Article11. Buist RJ, Deslauriers R, Saunders JK, Mainwood GW. 23Na and flame photometric studies of the NMR visibility of sodium in rat muscle. Can J Physiol Pharmacol. 1991; 69:1663–1669.
Article12. Zhang X, Ugurbil K, Chen W. Microstrip RF surface coil design for extremely high-field MRI and spectroscopy. Magn Reson Med. 2001; 46:443–450.
Article13. Jasinski K, Mlynarczyk A, Latta P, Volotovskyy V, Weglarz WP, Tomanek B. A volume microstrip RF coil for MRI microscopy. Magn Reson Imaging. 2012; 30:70–77.14. Fujita H. New horizons in MR technology: RF coil designs and trends. Magn Reson Med Sci. 2007; 6:29–42.
Article15. Rutledge O, Kwak T, Cao P, Zhang X. Design and test of a double-nuclear RF coil for (1)H MRI and (13)C MRSI at 7T. J Magn Reson. 2016; 267:15–21.16. Nielles-Vallespin S, Weber MA, Bock M, et al. 3D radial projection technique with ultrashort echo times for sodium MRI: clinical applications in human brain and skeletal muscle. Magn Reson Med. 2007; 57:74–81.
Article17. Kharrazian R, Jakob PM. Dynamics of 23Na during completely balanced steady-state free precession. J Magn Reson. 2006; 179:73–84.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Review on the RF Coil Designs and Trends for Ultra High Field Magnetic Resonance Imaging
- Analysis of Endcap Effect for MRI Birdcage RF Coil by FDTD Method
- Improvement of a 4-Channel Spiral-Loop RF Coil Array for TMJ MR Imaging at 7T
- Advances in Fast Vessel-Wall Magnetic Resonance Imaging Using High-Density Coil Arrays
- Dual Birdcage RF Coil for Leg MR Angiography