J Korean Soc Radiol.
2018 Feb;78(2):120-129. 10.3348/jksr.2018.78.2.120.
Transcatheter Arterial Embolization for Acute Arterial Extravasation with Hematoma Formation: Classified the Group as Cause and Their Clinical Outcomes
- Affiliations
-
- 1Department of Radiology, Chonbuk National University Medical School and Hospital, Jeonju, Korea. ymhan@jbnu.ac.kr
- 2Research Institute of Clinical Medicine, Chonbuk National University Medical School and Hospital, Jeonju, Korea.
- 3Institute of Cardiovascular Research, Chonbuk National University Medical School and Hospital, Jeonju, Korea.
- KMID: 2408262
- DOI: http://doi.org/10.3348/jksr.2018.78.2.120
Abstract
- PURPOSE
To present our experience in transcatheter arterial embolization (TAE) for hematoma formation related to variable causes. We analyzed the factors that could affect clinical outcomes.
MATERIALS AND METHODS
A retrospective study was conducted on 50 patients (24 men, 36 women; mean age, 63.8 years) who were treated for a TAE to control bleeding. Computed tomography (CT) scans showed the formation of hematomas. We classified the patients into three groups depending on the underlying cause of the hematoma i.e., spontaneous, traumatic or iatrogenic groups. We evaluated relevant factors such as sex, age, hematoma size and liquefaction, extravasation on CT, injured artery, onset to procedure time, embolization material, hospital day.
RESULTS
TAE was successfully performed in all patients. The proportions of patients in the spontaneous, traumatic, and iatrogenic bleeding groups were 36% (18/50), 42% (21/50), and 22% (11/50), respectively. Using the Mann Whitney U test, the international normalized ratio (INR) was statistically different for the spontaneous bleeding group (p = 0.013). In addition, the INR (p = 0.038) and platelet count (p = 0.004) were significant different for the traumatic group. Also, the platelet counts were related to clinical successes (p = 0.046).
CONCLUSION
Based our experience, TAE is a safe and effective treatment option for the management of hematoma formation. Furthermore, the interventional radiologist should consider the cause of hematoma formation in order to perform proper treatment.
MeSH Terms
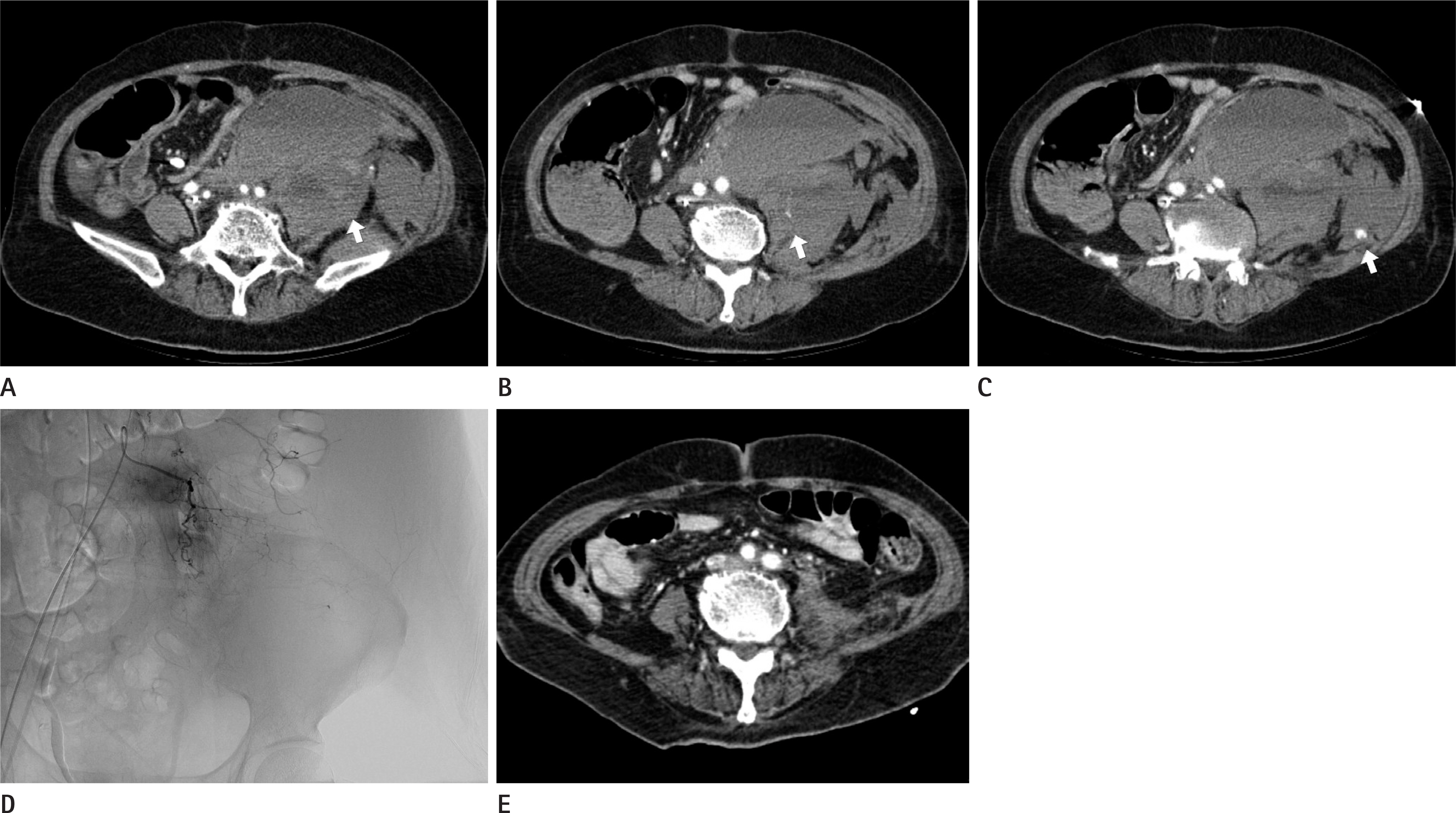
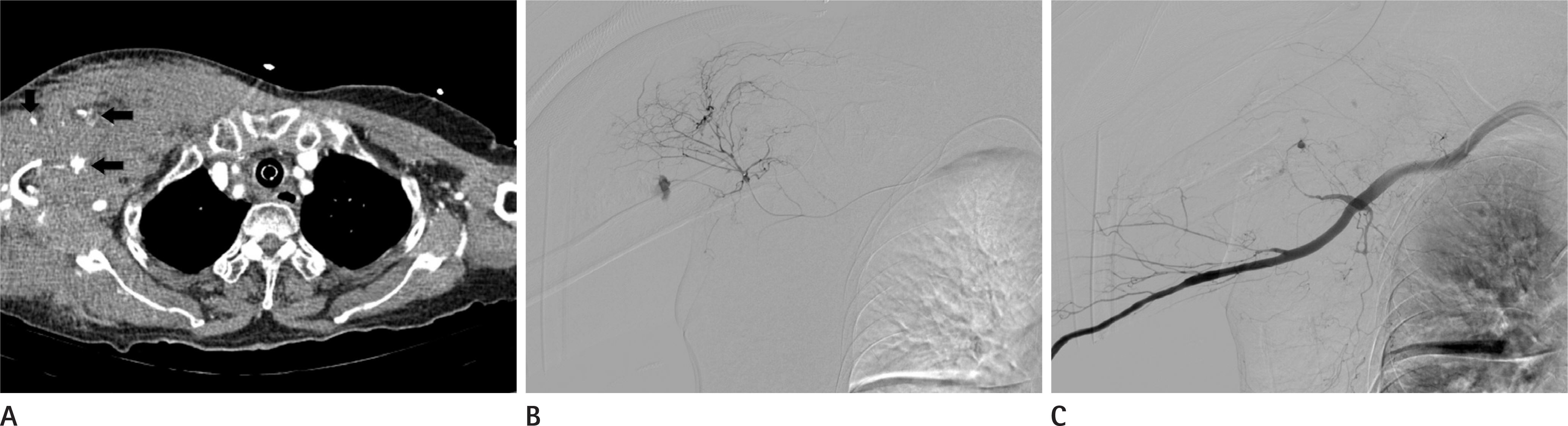
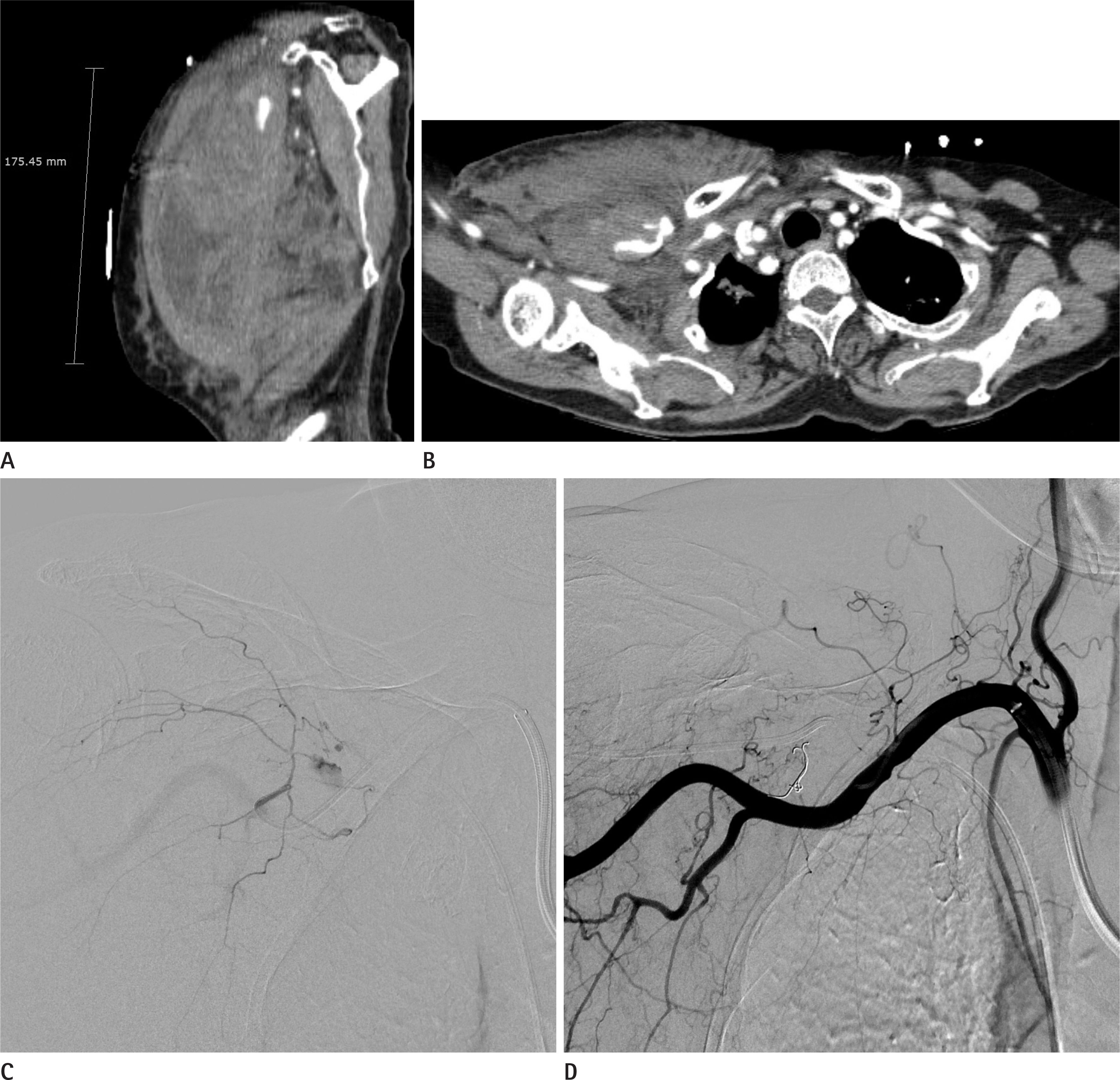
Figure
Reference
-
References
1. Hyers TM. Management of venous thromboembolism: past, present, and future. Arch Intern Med. 2003; 163:759–768.2. Boccardo P, Remuzzi G, Galbusera M. Platelet dysfunction in renal failure. Semin Thromb Hemost. 2004; 30:579–589.
Article3. Kaw D, Malhotra D. Platelet dysfunction and end-stage renal disease. Semin Dial. 2006; 19:317–322.4. Sunga KL, Bellolio MF, Gilmore RM, Cabrera D. Spontaneous retroperitoneal hematoma: etiology, characteristics, management, and outcome. J Emerg Med. 2012; 43:e157–e161.
Article5. Palatucci V, Lombardi G, Lombardi L, Giglio F, Giordano F, Lombardi D. Spontaneous muscle haematomas: management of 10 cases. Transl Med UniSa. 2014; 10:13–17.6. Minokadeh A, Pinsky MR. Postoperative hemodynamic instability and monitoring. Curr Opin Crit Care. 2016; 22:393–400.
Article7. Gando S, Hayakawa M. Pathophysiology of trauma-induced coagulopathy and management of critical bleeding requiring massive transfusion. Semin Thromb Hemost. 2016; 42:155–165.
Article8. Ko HK, Lee DY, Shim WH, Jang BC, Yoon CS, Won JH, et al. Endovascular treatment of abdominal aortic aneurysm by bifurcated stent graft. J Korean Radiol Soc. 1999; 41:909–914.
Article9. Li WH, Cheuk EC, Kowk PC, Cheung MT. Survival after transarterial embolization for spontaneous ruptured hepatocellular carcinoma. J Hepatobiliary Pancreat Surg. 2009; 16:508–512.
Article10. Cantón G, Levy DI, Lasheras JC, Nelson PK. Flow changes caused by the sequential placement of stents across the neck of sidewall cerebral aneurysms. J Neurosurg. 2005; 103:891–902.
Article11. Strate LL, Gralnek IM. ACG Clinical Guideline: management of patients with acute lower gastrointestinal bleeding. Am J Gastroenterol. 2016; 111:459–474.
Article12. Vaidya S, Tozer KR, Chen J. An overview of embolic agents. Semin Intervent Radiol. 2008; 25:204–215.
Article13. Del Cura JL, Zabala R, Cotra I. [Ultrasound-guided interventional procedures in the musculoskeletal system]. Radiolo-gia. 2010; 52:525–533.
Article14. Song JS, Kwak HS, Han YM. Transarterial embolization of arterial bleeding in patients with pelvic bone fractures. J Korean Soc Radiol. 2009; 61:311–318.
Article15. Akpinar E, Peynircioglu B, Turkbey B, Cil BE, Balkanci F. Endovascular management of life-threatening retroperitoneal bleeding. ANZ J Surg. 2008; 78:683–687.
Article16. Jain V, Ganpule A, Vyas J, Muthu V, Sabnis RB, Rajapurkar MM, et al. Management of non-neoplastic renal hemorrhage by transarterial embolization. Urology. 2009; 74:522–526.
Article17. Beaujeux R, Saussine C, al-Fakir A, Boudjema K, Roy C, Jac-qmin D, et al. Superselective endovascular treatment of renal vascular lesions. J Urol. 1995; 153:14–17.
Article18. Alesawi AM, El-Hakim A, Zorn KC, Saad F. Radiation-induced hemorrhagic cystitis. Curr Opin Support Palliat Care. 2014; 8:235–240.
Article19. Dutta S, Sanjay P, Jones ML. Diagnosis and treatment of giant lateral abdominal wall haematoma after blunt trauma: a case report. Cases J. 2009; 2:9358.
Article20. Park JY, Yim NY, Kim JK, Kim HO, Kang YJ, Jung HD, et al. Embolization of trauma-associated pelvic hemorrhage: feasibility of superselective catheterization in heavily injured patients as a damage control for life-threatening pelvic bleeding. J Korean Soc Radiol. 2016; 74:236–244.
Article21. Cho YH, Lee HS, Kim HC, Kim SG, Chung TW, Kim YH, et al. Transcatheter arterial embolizations of arterial bleeding in patients with pelvic bone fracture. J Korean Radiol Soc. 1999; 41:903–908.
Article22. Zissin R, Gayer G, Kots E, Ellis M, Bartal G, Griton I. Transcatheter arterial embolisation in anticoagulant-related haematoma–a current therapeutic option: a report of four patients and review of the literature. Int J Clin Pract. 2007; 61:1321–1327.23. Lai BM, Shum JS, Chu CY, Lo SS, Lau KY. Predictors of the success and failure of emergency pelvic artery embolisation for primary postpartum haemorrhage: a 12-year review. Singapore Med J. 2017; 58:272–278.
Article24. Agolini SF, Shah K, Jaffe J, Newcomb J, Rhodes M, Reed JF 3rd. Arterial embolization is a rapid and effective technique for controlling pelvic fracture hemorrhage. J Trauma. 1997; 43:395–399.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Outcomes and complications of embolization for gastrointestinal bleeding
- Transcatheter hepatic arterial embolization of ruptured hepatoma with massive intraperitoneal bleeding
- Transcatheter arterial embolization for congenital renal arteriovenous fistula
- Selective Arterial Embolization of Renal Arteriovenous Fistula and Arterial Aneurysm
- Endovascular treatment of pancreatitis-related gastrointestinal bleeding