Korean Circ J.
2018 Apr;48(4):296-309. 10.4070/kcj.2017.0119.
β-arrestin2 Affects Cardiac Progenitor Cell Survival through Cell Mobility and Tube Formation in Severe Hypoxia
- Affiliations
-
- 1Department of Internal Medicine, Chungbuk National University College of Medicine, Cheongju, Korea. drcorazon@hanmail.net
- 2Chungbuk Regional Cardiocerebrovascular Center, Chungbuk National University Hospital, Cheongju, Korea.
- 3Center for Translational Medicine, Department of Pharmacology, Temple University Lewis Katz School of Medicine, Philadelphia, PA, USA.
- KMID: 2407899
- DOI: http://doi.org/10.4070/kcj.2017.0119
Abstract
- BACKGROUND AND OBJECTIVES
β-arrestin2 (β-arr2) basically regulates multiple signaling pathways in mammalian cells by desensitization and internalization of G-protein coupled receptors (GPCRs). We investigated impacts of β-arr2 on survival, mobility, and tube formation of cardiac progenitor cells (CPCs) obtained from wild-type (WT) mouse (CPC-WT), and β-arr2 knock-out (KO) mouse (CPC-KO) cultured in presence or absence of serum and oxygen as non-canonical roles in GPCR system.
METHODS
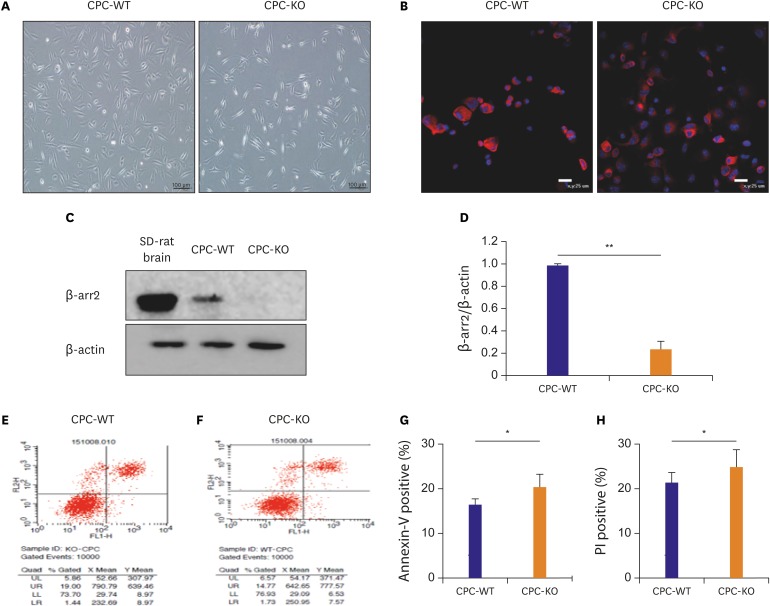
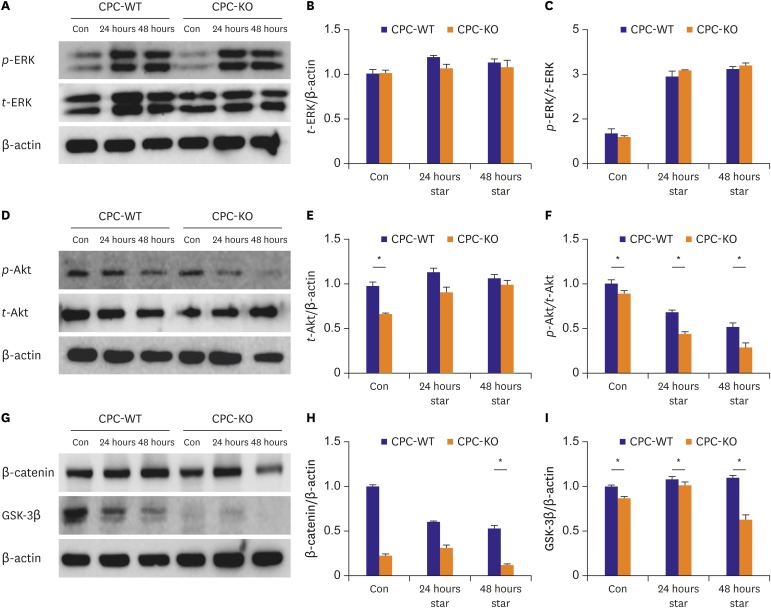
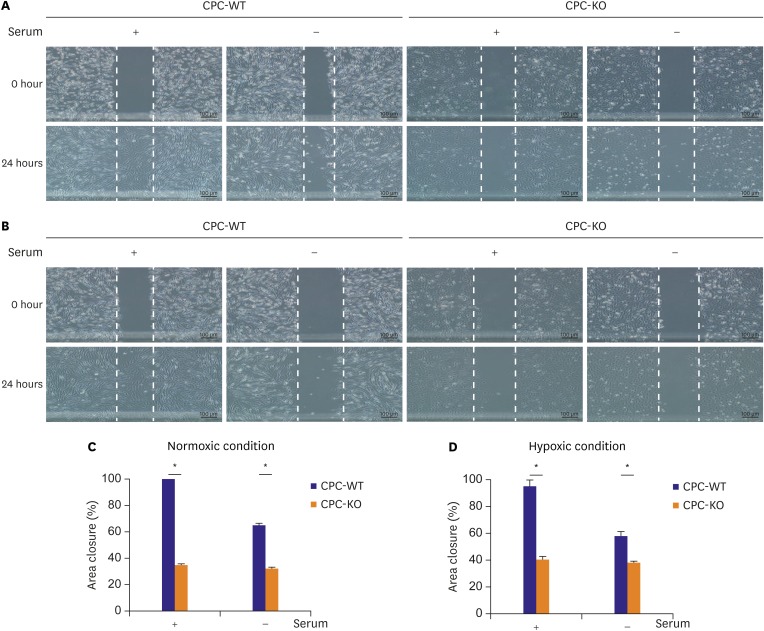
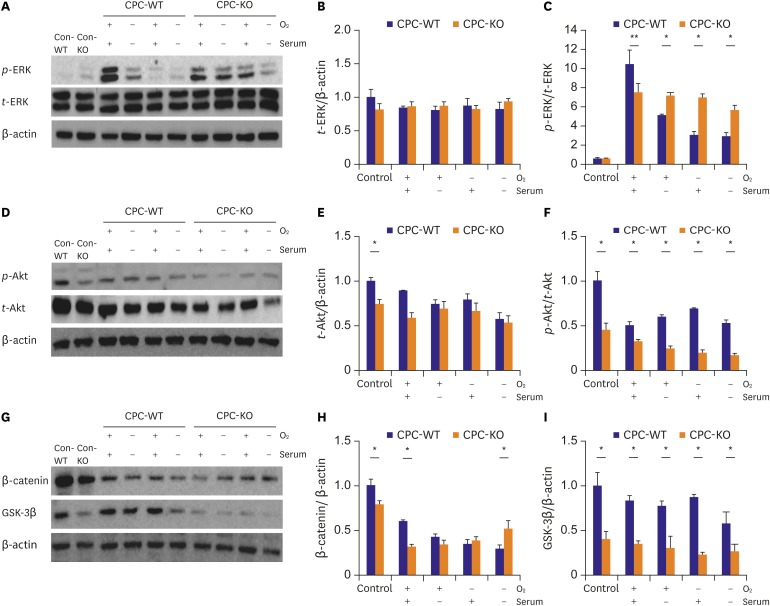
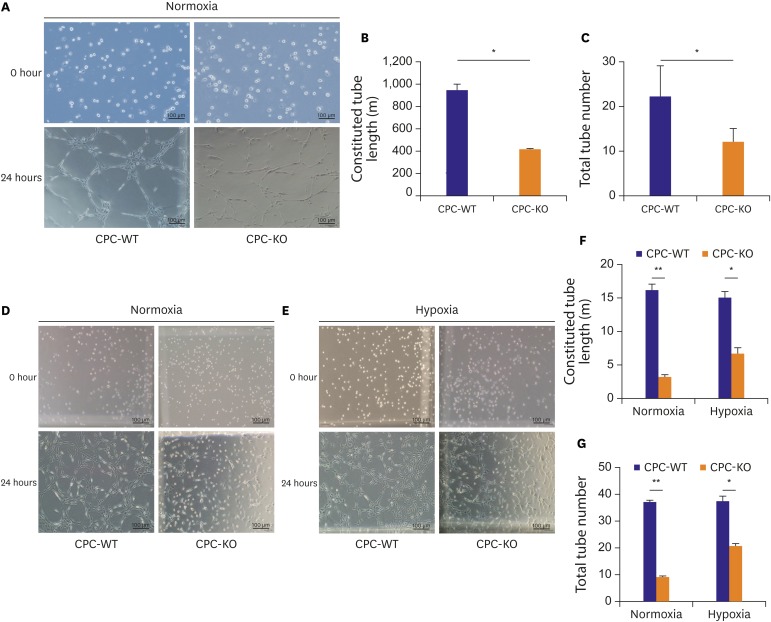
CPCs were cultured in Dulbecco's Modified Eagle Medium/Nutrient Mixture F-12 -based media containing fetal bovine serum and growth factors. Survival of 2 types of CPCs in hypoxia and/or serum deprivation was measured by fluorescence-activated cell sorting. Wound healing ability, and tube formation ability on Matrigel of 2 kinds of CPCs were compared in normoxic and hypoxic cultures. Protein expression related to survival and mobility were measured with the Western blot for each culture conditions. RESULT: CPC-KO showed significantly worse mobility in the wound healing assay and in tube formation on Matrigel especially in hypoxic culture than did the CPC-WT. Also, CPC-KO showed significantly higher apoptosis fraction in both normoxic and hypoxic cultures than did the CPC-WT. Expression of proteins associated with cell survival and mobility, e.g., protein kinase B (Akt), β-catenin, and glycogen synthase kinase-3β (GSK-3β) was significantly worse in CPC-KO.
CONCLUSIONS
The CPC-KO had significantly worse cell mobility, tube formation ability, and survival than the CPC-WT, especially in the hypoxic cultures. Apparently, β-arr2 is important on CPC survival by means of mobility and tube formation in myocardial ischemia.
MeSH Terms
-
Animals
Anoxia*
Apoptosis
Blotting, Western
Cell Movement
Cell Survival
Eagles
Flow Cytometry
Glycogen Synthase
GTP-Binding Proteins
Intercellular Signaling Peptides and Proteins
Mice
Myocardial Ischemia
Oxygen
Proto-Oncogene Proteins c-akt
Stem Cells*
Wound Healing
GTP-Binding Proteins
Glycogen Synthase
Intercellular Signaling Peptides and Proteins
Oxygen
Proto-Oncogene Proteins c-akt
Figure
Cited by 1 articles
-
You're Not under Arrest: Worry-free with β-arrestin
Jubert Marquez, Jin Han
Korean Circ J. 2018;48(4):325-328. doi: 10.4070/kcj.2018.0057.
Reference
-
1. Youn JC, Han S, Ryu KH. Temporal trends of hospitalized patients with heart failure in Korea. Korean Circ J. 2017; 47:16–24. PMID: 28154584.2. Lee JH, Lim NK, Cho MC, Park HY. Epidemiology of heart failure in Korea: present and future. Korean Circ J. 2016; 46:658–664. PMID: 27721857.3. Liang SX, Phillips WD. Migration of resident cardiac stem cells in myocardial infarction. Anat Rec (Hoboken). 2013; 296:184–191. PMID: 23225361.4. Beltrami AP, Barlucchi L, Torella D, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003; 114:763–776. PMID: 14505575.5. Kuang D, Zhao X, Xiao G, et al. Stem cell factor/c-kit signaling mediated cardiac stem cell migration via activation of p38 MAPK. Basic Res Cardiol. 2008; 103:265–273. PMID: 18087667.6. Lefkowitz RJ, Rajagopal K, Whalen EJ. New roles for β-arrestin in cell signaling: not just for seven-transmembrane receptors. Mol Cell. 2006; 24:643–652. PMID: 17157248.7. DeFea KA. Arrestins in actin reorganization and cell migration. Prog Mol Biol Transl Sci. 2013; 118:205–222. PMID: 23764055.8. Yang P, Li Z, Wang Y, Zhang L, Wu H, Li Z. Secreted pyruvate kinase M2 facilitates cell migration via PI3K/Akt and Wnt/β-catenin pathway in colon cancer cells. Biochem Biophys Res Commun. 2015; 459:327–332. PMID: 25732087.9. Traynham CJ, Hullmann J, Koch WJ. Canonical and non-canonical actions of GRK5 in the heart. J Mol Cell Cardiol. 2016; 92:196–202. PMID: 26829117.10. Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin FT. Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science. 1999; 286:2495–2498. PMID: 10617462.11. Attramadal H, Arriza JL, Aoki C, et al. Beta-arrestin2, a novel member of the arrestin/beta-arrestin gene family. J Biol Chem. 1992; 267:17882–17890. PMID: 1517224.12. Fransioli J, Bailey B, Gude NA, et al. Evolution of the c-kit-positive cell response to pathological challenge in the myocardium. Stem Cells. 2008; 26:1315–1324. PMID: 18308948.13. Chun WJ, Nah DY, Bae JH, Chung JW, Lee H, Moon IS. Glucose-insulin-potassium solution protects ventricular myocytes of neonatal rat in an in vitro coverslip ischemia/reperfusion model. Korean Circ J. 2015; 45:234–241. PMID: 26023312.14. Naaldijk Y, Johnson AA, Ishak S, Meisel HJ, Hohaus C, Stolzing A. Migrational changes of mesenchymal stem cells in response to cytokines, growth factors, hypoxia, and aging. Exp Cell Res. 2015; 338:97–104. PMID: 26335540.15. Park KJ, Kim YJ, Choi EJ, et al. Expression pattern of the thioredoxin system in human endothelial progenitor cells and endothelial cells under hypoxic injury. Korean Circ J. 2010; 40:651–658. PMID: 21267388.16. Takeda N, Manabe I. Cellular interplay between cardiomyocytes and nonmyocytes in cardiac remodeling. Int J Inflamm. 2011; 2011:535241.17. Williams AR, Hatzistergos KE, Addicott B, et al. Enhanced effect of combining human cardiac stem cells and bone marrow mesenchymal stem cells to reduce infarct size and to restore cardiac function after myocardial infarction. Circulation. 2013; 127:213–223. PMID: 23224061.18. Makkar RR, Smith RR, Cheng K, et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet. 2012; 379:895–904. PMID: 22336189.19. van Berlo JH, Kanisicak O, Maillet M, et al. c-kit+ cells minimally contribute cardiomyocytes to the heart. Nature. 2014; 509:337–341. PMID: 24805242.20. Luttrell LM, Lefkowitz RJ. The role of beta-arrestins in the termination and transduction of G-protein-coupled receptor signals. J Cell Sci. 2002; 115:455–465. PMID: 11861753.21. Buchanan FG, DuBois RN. Emerging roles of beta-arrestins. Cell Cycle. 2006; 5:2060–2063. PMID: 16969081.22. Walker JK, Fong AM, Lawson BL, et al. β-Arrestin-2 regulates the development of allergic asthma. J Clin Invest. 2003; 112:566–574. PMID: 12925697.23. Ge L, Ly Y, Hollenberg M, DeFea K. A beta-arrestin-dependent scaffold is associated with prolonged MAPK activation in pseudopodia during protease-activated receptor-2-induced chemotaxis. J Biol Chem. 2003; 278:34418–34426. PMID: 12821670.24. McCubrey JA, Steelman LS, Bertrand FE, et al. Multifaceted roles of GSK-3 and Wnt/β-catenin in hematopoiesis and leukemogenesis: opportunities for therapeutic intervention. Leukemia. 2014; 28:15–33. PMID: 23778311.25. Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005; 434:843–850. PMID: 15829953.26. Mannoury la Cour C, Salles MJ, Pasteau V, Millan MJ. Signaling pathways leading to phosphorylation of Akt and GSK-3β by activation of cloned human and rat cerebral D2 and D3 receptors. Mol Pharmacol. 2011; 79:91–105. PMID: 20952497.27. Krock BL, Skuli N, Simon MC. Hypoxia-induced angiogenesis: good and evil. Genes Cancer. 2011; 2:1117–1133. PMID: 22866203.28. Zou L, Yang R, Chai J, Pei G. Rapid xenograft tumor progression in beta-arrestin1 transgenic mice due to enhanced tumor angiogenesis. FASEB J. 2008; 22:355–364. PMID: 17890288.29. Zhang T, Lee YW, Rui YF, Cheng TY, Jiang XH, Li G. Bone marrow-derived mesenchymal stem cells promote growth and angiogenesis of breast and prostate tumors. Stem Cell Res Ther. 2013; 4:70. PMID: 23763837.30. Takeda K, Sowa Y, Nishino K, Itoh K, Fushiki S. Adipose-derived stem cells promote proliferation, migration, and tube formation of lymphatic endothelial cells in vitro by secreting lymphangiogenic factors. Ann Plast Surg. 2015; 74:728–736. PMID: 24401810.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- High Glucose Causes Human Cardiac Progenitor Cell Dysfunction by Promoting Mitochondrial Fission: Role of a GLUT1 Blocker
- The Role of Hypoxia in Brain Tumor Immune Responses
- Roles of Dopamine D₂ Receptor Subregions in Interactions with β-Arrestin2
- Hypoxia-dependent mitochondrial fission regulates endothelial progenitor cell migration, invasion, and tube formation
- Molecular Signature That Determines the Acute Tolerance of G Protein-Coupled Receptors