Korean Circ J.
2018 Apr;48(4):287-295. 10.4070/kcj.2017.0342.
Laboratory Markers in Incomplete Kawasaki Disease according to Coronary Artery Outcome
- Affiliations
-
- 1Department of Pediatrics, Korea University Guro Hospital, Seoul, Korea. leejmd@chol.com
- 2Department of Pediatrics, Korea University Ansan Hospital, Seoul, Korea.
- 3Department of Pediatrics, Korea University Anam Hospital, Seoul, Korea.
- KMID: 2407898
- DOI: http://doi.org/10.4070/kcj.2017.0342
Abstract
- BACKGROUND AND OBJECTIVES
We defined laboratory marker profiles typical of incomplete Kawasaki disease (iKD) during illness, especially with respect to the presence of a coronary artery abnormality such as coronary artery dilation or aneurysm.
METHODS
This retrospective study examined the clinical and laboratory markers of patients with iKD over time, along with those of patients with complete KD (cKD) and febrile controls.
RESULTS
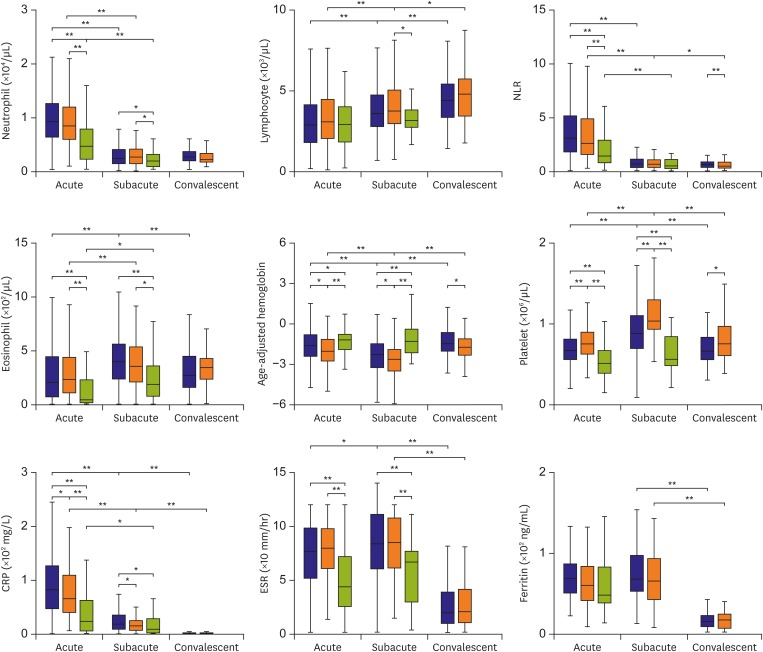
Of 795 patients, 178 had iKD, 504 had cKD and 113 were febrile controls. During the transition from the acute to subacute phase, the age-adjusted hemoglobin levels and platelet counts were significantly lower and higher, respectively, in the subacute phase than in the acute phase in both iKD and cKD patients, which differed from those of febrile controls. Lower levels of acute and subacute age-adjusted hemoglobin levels in iKD patients (odds ratio [OR], 0.538 and 0.583; p=0.006 and 0.018, respectively) and higher subacute platelet counts in cKD patients (OR, 1.004; p=0.014) were correlated with the risk of coronary dilation. A higher acute neutrophil-to-lymphocyte ratio was associated with aneurysm only in cKD patients (OR, 1.059; p=0.044).
CONCLUSIONS
The iKD patients share KD-specific laboratory marker profiles in terms of complete blood cell counts and acute phase reactant levels with cKD patients. However, the factors predicting coronary dilation differ according to the phenotype; lower acute and subacute age-adjusted hemoglobin levels predict coronary dilation only in iKD patients.
MeSH Terms
Figure
Cited by 1 articles
-
Risk Factors Related to Coronary Artery Outcome in Kawasaki Disease
Soo-Jin Kim
Korean Circ J. 2018;48(4):329-331. doi: 10.4070/kcj.2018.0077.
Reference
-
1. Newburger JW, Takahashi M, Gerber MA, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 2004; 110:2747–2771. PMID: 15505111.
Article2. Yellen ES, Gauvreau K, Takahashi M, et al. Performance of 2004 American Heart Association recommendations for treatment of Kawasaki disease. Pediatrics. 2010; 125:e234–e241. PMID: 20100771.
Article3. Manlhiot C, Christie E, McCrindle BW, Rosenberg H, Chahal N, Yeung RS. Complete and incomplete Kawasaki disease: two sides of the same coin. Eur J Pediatr. 2012; 171:657–662. PMID: 22134803.
Article4. Song D, Yeo Y, Ha K, et al. Risk factors for Kawasaki disease-associated coronary abnormalities differ depending on age. Eur J Pediatr. 2009; 168:1315–1321. PMID: 19159953.
Article5. Sudo D, Monobe Y, Yashiro M, et al. Coronary artery lesions of incomplete Kawasaki disease: a nationwide survey in Japan. Eur J Pediatr. 2012; 171:651–656. PMID: 22159904.
Article6. Witt MT, Minich LL, Bohnsack JF, Young PC. Kawasaki disease: more patients are being diagnosed who do not meet American Heart Association criteria. Pediatrics. 1999; 104:e10. PMID: 10390296.
Article7. Zhang W, Li Q, Zhao XD, et al. Clinical analysis of 942 cases of Kawasaki disease. Zhonghua Er Ke Za Zhi. 2006; 44:324–328. PMID: 16780705.8. Tremoulet AH, Jain S, Chandrasekar D, Sun X, Sato Y, Burns JC. Evolution of laboratory values in patients with Kawasaki disease. Pediatr Infect Dis J. 2011; 30:1022–1026. PMID: 21817952.
Article9. Kawasaki T. Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children. Arerugi. 1967; 16:178–222. PMID: 6062087.10. Cha Y. Classification and diagnosis of red blood cell diseases. The Korean Society of Hematology. Hematology. 2nd ed. Seoul: Panmun Education;2011. p. 49.11. Japan Kawasaki Disease Research Committee. Report of subcommittee on standardization of diagnostic criteria and reporting of coronary artery lesions in Kawasaki disease. Tokyo: Ministry of Health and Welfare;1984.12. Comenzo RL, Malachowski ME, Meissner HC, Fulton DR, Berkman EM. Immune hemolysis, disseminated intravascular coagulation, and serum sickness after large doses of immune globulin given intravenously for Kawasaki disease. J Pediatr. 1992; 120:926–928. PMID: 1593353.
Article13. Kahwaji J, Barker E, Pepkowitz S, et al. Acute hemolysis after high-dose intravenous immunoglobulin therapy in highly HLA sensitized patients. Clin J Am Soc Nephrol. 2009; 4:1993–1997. PMID: 19833910.
Article14. Nemeth E, Ganz T. Anemia of inflammation. [vi.]. Hematol Oncol Clin North Am. 2014; 28:671–681. PMID: 25064707.
Article15. Sabharwal T, Manlhiot C, Benseler SM, et al. Comparison of factors associated with coronary artery dilation only versus coronary artery aneurysms in patients with Kawasaki disease. Am J Cardiol. 2009; 104:1743–1747. PMID: 19962487.
Article16. Ha KS, Lee J, Jang GY, et al. Value of neutrophil-lymphocyte ratio in predicting outcomes in Kawasaki disease. Am J Cardiol. 2015; 116:301–306. PMID: 25975725.
Article17. Terai M, Yasukawa K, Honda T, et al. Peripheral blood eosinophilia and eosinophil accumulation in coronary microvessels in acute Kawasaki disease. Pediatr Infect Dis J. 2002; 21:777–781. PMID: 12192168.
Article18. Ashouri N, Takahashi M, Dorey F, Mason W. Risk factors for nonresponse to therapy in Kawasaki disease. J Pediatr. 2008; 153:365–368. PMID: 18534243.
Article19. Egami K, Muta H, Ishii M, et al. Prediction of resistance to intravenous immunoglobulin treatment in patients with Kawasaki disease. J Pediatr. 2006; 149:237–240. PMID: 16887442.
Article20. Kobayashi T, Inoue Y, Takeuchi K, et al. Prediction of intravenous immunoglobulin unresponsiveness in patients with Kawasaki disease. Circulation. 2006; 113:2606–2612. PMID: 16735679.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diagnosis of incomplete Kawasaki disease
- CABG for an Adult with Coronary Disease due to Kawasaki Disease
- Percutaneous Transluminal Coronary Angioplasty for Coronary Artery Stenosis in an Adult Kawasaki Disease with Coronary Aneurysm : A Case Report and Review
- A Clinical Study of Atypical Kawasaki Disease: A Rate of Coronary Artery Involvement
- Changes in Coronary Perfusion after Occlusion of Coronary Arteries in Kawasaki Disease