Ann Rehabil Med.
2014 Apr;38(2):200-208.
Comparison of Treatment Effects Between Children With Spastic Cerebral Palsy Under and Over Five Years After Botulinum Toxin Type A Injection
- Affiliations
-
- 1Department of Rehabilitation Medicine, Catholic University of Daegu School of Medicine, Daegu, Korea. coolkwon@cu.ac.kr
Abstract
OBJECTIVE
To evaluate whether age influences a change in the spasticity of the ankle plantar flexor after botulinum toxin type A (BTA) injection in children with spastic cerebral palsy (CP).
METHODS
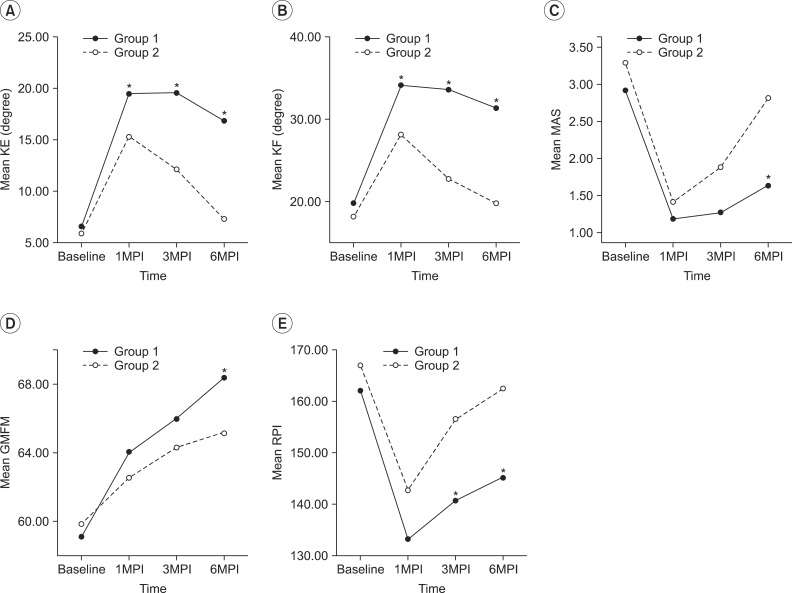
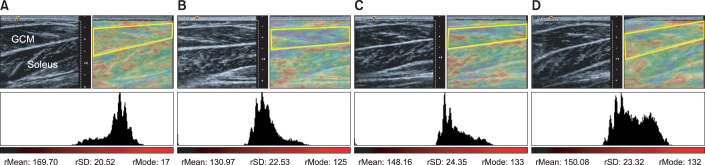
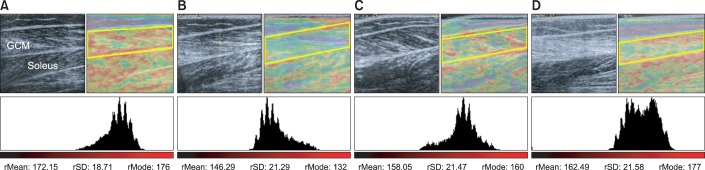
Sixteen children with spastic CP were enrolled in the study. Seven children (group 1) were under 5 years of age, and nine (group 2) were over 5 years of age. They all received BTA injection in the gastrocnemius muscle (GCM) under ultrasound guidance. Passive range of motion (PROM) of ankle dorsiflexion, Modified Ashworth Scale (MAS) of the ankle plantar flexor, Gross Motor Function Measure (GMFM) and median red pixel intensity (RPI) of the medial GCM on real-time sonoelastography were measured at baseline (pre-injection) and 1-, 3-, and 6-month post-injection.
RESULTS
In both groups, the mean PROM, MAS, and RPI were significantly improved after injection until 6-month post-injection. The change of PROM of ankle dorsiflexion in group 1 was significantly greater than that in group 2, until 6-month post-injection. The change in the MAS and GMFM between baseline and 6-month post-injection in group 1 was greater than that in group 2. The changes in the median RPI between baseline and 3- and 6-month post-injections were greater in group 1 than in group 2.
CONCLUSION
Our pilot study demonstrated the different changes in spasticity of the ankle plantar flexor after BTA injection based on age. Therefore, age may be considered when establishing a treatment plan using BTA injection for children with spastic CP.
MeSH Terms
Figure
Reference
-
1. Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther. 1987; 67:206–207. PMID: 3809245.
Article2. Gracies JM, Burke K, Clegg NJ, Browne R, Rushing C, Fehlings D, et al. Reliability of the Tardieu Scale for assessing spasticity in children with cerebral palsy. Arch Phys Med Rehabil. 2010; 91:421–428. PMID: 20298834.
Article3. Dietz V, Sinkjaer T. Spastic movement disorder: impaired reflex function and altered muscle mechanics. Lancet Neurol. 2007; 6:725–733. PMID: 17638613.
Article4. Booth CM, Cortina-Borja MJ, Theologis TN. Collagen accumulation in muscles of children with cerebral palsy and correlation with severity of spasticity. Dev Med Child Neurol. 2001; 43:314–320. PMID: 11368484.
Article5. Kim K, Shin HI, Kwon BS, Kim SJ, Jung IY, Bang MS. Neuronox versus BOTOX for spastic equinus gait in children with cerebral palsy: a randomized, double-blinded, controlled multicentre clinical trial. Dev Med Child Neurol. 2011; 53:239–244. PMID: 21087238.
Article6. Ade-Hall RA, Moore AP. Botulinum toxin type A in the treatment of lower limb spasticity in cerebral palsy. Cochrane Database Syst Rev. 2000; (2):CD001408. PMID: 10796784.
Article7. Cosgrove AP, Corry IS, Graham HK. Botulinum toxin in the management of the lower limb in cerebral palsy. Dev Med Child Neurol. 1994; 36:386–396. PMID: 8168657.
Article8. Borodic GE, Ferrante R, Pearce LB, Smith K. Histologic assessment of dose-related diffusion and muscle fiber response after therapeutic botulinum A toxin injections. Mov Disord. 1994; 9:31–39. PMID: 8139603.
Article9. Park GY, Kwon DR. Application of real-time sonoelastography in musculoskeletal diseases related to physical medicine and rehabilitation. Am J Phys Med Rehabil. 2011; 90:875–886. PMID: 21552109.
Article10. Kwon DR, Park GY, Lee SU, Chung I. Spastic cerebral palsy in children: dynamic sonoelastographic findings of medial gastrocnemius. Radiology. 2012; 263:794–801. PMID: 22495685.
Article11. Park GY, Kwon DR. Sonoelastographic evaluation of medial gastrocnemius muscles intrinsic stiffness after rehabilitation therapy with botulinum toxin a injection in spastic cerebral palsy. Arch Phys Med Rehabil. 2012; 93:2085–2089. PMID: 22776155.
Article12. Kwon DR, Park GY, Kwon JG. The change of intrinsic stiffness in gastrocnemius after intensive rehabilitation with botulinum toxin A injection in spastic diplegic cerebral palsy. Ann Rehabil Med. 2012; 36:400–403. PMID: 22837977.
Article13. de Paiva A, Meunier FA, Molgo J, Aoki KR, Dolly JO. Functional repair of motor endplates after botulinum neurotoxin type A poisoning: biphasic switch of synaptic activity between nerve sprouts and their parent terminals. Proc Natl Acad Sci U S A. 1999; 96:3200–3205. PMID: 10077661.
Article14. Pierce SR, Prosser LA, Lauer RT. Relationship between age and spasticity in children with diplegic cerebral palsy. Arch Phys Med Rehabil. 2010; 91:448–451. PMID: 20298838.
Article15. Pascual-Pascual SI, Pascual-Castroviejo I, Ruiz PJ. Treating spastic equinus foot from cerebral palsy with botulinum toxin type A: what factors influence the results?: an analysis of 189 consecutive cases. Am J Phys Med Rehabil. 2011; 90:554–563. PMID: 21765274.16. Wang Y, Gao B. A dose-response relationship research on botulinum toxin type A local intramuscular injections of lower extremity spasticity in children with cerebral palsy. Childs Nerv Syst. 2008; 24:545–547. PMID: 18297290.
Article17. Dietz V, Trippel M, Berger W. Reflex activity and muscle tone during elbow movements in patients with spastic paresis. Ann Neurol. 1991; 30:767–779. PMID: 1789693.
Article18. Sommerfeld DK, Gripenstedt U, Welmer AK. Spasticity after stroke: an overview of prevalence, test instruments, and treatments. Am J Phys Med Rehabil. 2012; 91:814–820. PMID: 22760104.19. Zimmerman SD, McCormick RJ, Vadlamudi RK, Thomas DP. Age and training alter collagen characteristics in fast- and slow-twitch rat limb muscle. J Appl Physiol (1985). 1993; 75:1670–1674. PMID: 8282619.
Article20. Friden J, Lieber RL. Spastic muscle cells are shorter and stiffer than normal cells. Muscle Nerve. 2003; 27:157–164. PMID: 12548522.
Article21. Feit H, Kawai M, Mostafapour AS. Increased resistance of the collagen in avian dystrophic muscle to collagenolytic attack: evidence for increased crosslinking. Muscle Nerve. 1989; 12:476–485. PMID: 2542788.
Article22. Coutinho EL, DeLuca C, Salvini TF, Vidal BC. Bouts of passive stretching after immobilization of the rat soleus muscle increase collagen macromolecular organization and muscle fiber area. Connect Tissue Res. 2006; 47:278–286. PMID: 17118750.
Article23. Rosenbaum PL, Walter SD, Hanna SE, Palisano RJ, Russell DJ, Raina P, et al. Prognosis for gross motor function in cerebral palsy: creation of motor development curves. JAMA. 2002; 288:1357–1363. PMID: 12234229.
Article24. Harries N, Kassirer M, Amichai T, Lahat E. Changes over years in gross motor function of 3-8 year old children with cerebral palsy: using the Gross Motor Function Measure (GMFM-88). Isr Med Assoc J. 2004; 6:408–411. PMID: 15274531.25. Beckung E, Carlsson G, Carlsdotter S, Uvebrant P. The natural history of gross motor development in children with cerebral palsy aged 1 to 15 years. Dev Med Child Neurol. 2007; 49:751–756. PMID: 17880644.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Botulinum Toxin A Treatment in Cerebral Palsy
- Electrophysiological Changes after Botulinum Toxin Type A in Children with Cerebral Palsy
- Botulinum Toxin Treatment on Upper Limb Function in School Age Children With Bilateral Spastic Cerebral Palsy: One Year Follow-up
- The Change of Intrinsic Stiffness in Gastrocnemius after Intensive Rehabilitation with Botulinum Toxin A Injection in Spastic Diplegic Cerebral Palsy
- Treatment Effects of Botulinum Toxin A in Cerebral Palsy with Foot Deformities