Yonsei Med J.
2018 May;59(3):452-456. 10.3349/ymj.2018.59.3.452.
Safety of Tocilizumab in Rheumatoid Arthritis Patients with Resolved Hepatitis B Virus Infection: Data from Real-World Experience
- Affiliations
-
- 1Division of Rheumatology, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea. sangwonlee@yuhs.ac
- 2Division of Gastroenterology, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea. drpjy@yuhs.ac
- 3Yonsei Liver Center, Severance Hospital, Seoul, Korea.
- 4Institute for Immunology and Immunological Diseases, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2407870
- DOI: http://doi.org/10.3349/ymj.2018.59.3.452
Abstract
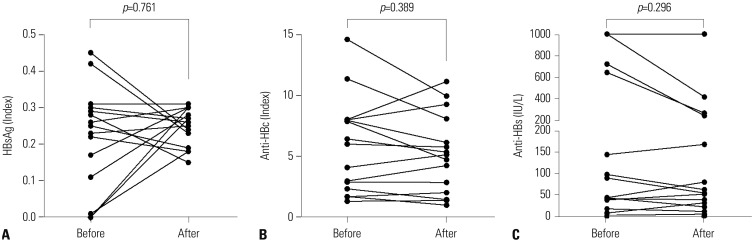
- To investigate whether the use of IL-6 receptor antagonist (tocilizumab) might be associated with hepatitis B virus (HBV) reactivation in rheumatoid arthritis (RA) patients, particularly in those with resolved HBV infection [HBV surface antigen (HBsAg) negative and antibody to HBV core antigen (anti-HBc) positive, serologically]. HBsAg, anti-HBc, antibody to HBsAg (anti-HBs), and HBV DNA titers were measured in RA patients who had continuously received tocilizumab for more than 3 months. Patients were divided into two groups according to the presence of anti-HBc. Clinical and laboratory data, in addition to medications administered along with tocilizumab during the treatment duration with tocilizumab, were compared between the two groups. HBV reactivation was defined as the presence of HBV DNA in sera, and alterations in HBsAg, anti-HBc, and anti-HBs titers according to the use of tocilizumab were also evaluated. Fifteen of 39 patients (38.5%) had anti-HBc positivity, while 24 patients (61.5%) did not. There were no differences in demographic data, serologic classification, and variables related to tocilizumab between the anti-HBc-positive and -negative groups. Comparison of the medications administered along with tocilizumab treatment revealed no meaningful differences. None of the patients experienced reactivation of HBV. In addition, in 15 patients with resolved HBV infection, no alterations in HBsAg, anti-HBc, and anti-HBs titers were observed with the use of tocilizumab. Tocilizumab may be applied to RA patients safely with few concerns for HBV reactivation, particularly in those with resolved HBV infection.
Keyword
MeSH Terms
Figure
Reference
-
1. Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016; 388:2023–2038. PMID: 27156434.
Article2. McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011; 365:2205–2219. PMID: 22150039.
Article3. Smolen JS, Landewé R, Bijlsma J, Burmester G, Chatzidionysiou K, Dougados M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis. 2017; 76:960–977. PMID: 28264816.4. Harrold LR, Peterson D, Beard AJ, Gurwitz JH, Briesacher BA. Time trends in medication use and expenditures in older patients with rheumatoid arthritis. Am J Med. 2012; 125:937.e9–937.e15.
Article5. Trépo C, Chan HL, Lok A. Hepatitis B virus infection. Lancet. 2014; 384:2053–2063. PMID: 24954675.
Article6. Loomba R, Liang TJ. Hepatitis B reactivation associated with immune suppressive and biological modifier therapies: current concepts, management strategies, and future directions. Gastroenterology. 2017; 152:1297–1309. PMID: 28219691.
Article7. Hoofnagle JH. Reactivation of hepatitis B. Hepatology. 2009; 49(5 Suppl):S156–S165. PMID: 19399803.
Article8. Lee JI. Reactivation of hepatitis B virus in patients with rheumatologic disease treated with biologic disease modifying anti-rheumatic drugs: screening and treatment. J Rheum Dis. 2015; 22:282–287.
Article9. Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010; 62:2569–2581. PMID: 20872595.
Article10. Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis. 2010; 69:1580–1588. PMID: 20699241.
Article11. Nakamura J, Nagashima T, Nagatani K, Yoshio T, Iwamoto M, Minota S. Reactivation of hepatitis B virus in rheumatoid arthritis patients treated with biological disease-modifying antirheumatic drugs. Int J Rheum Dis. 2016; 19:470–475. PMID: 24698305.
Article12. Mochida S, Nakao M, Nakayama N, Uchida Y, Nagoshi S, Ido A, et al. Nationwide prospective and retrospective surveys for hepatitis B virus reactivation during immunosuppressive therapies. J Gastroenterol. 2016; 51:999–1010. PMID: 26831356.
Article13. Kato M, Atsumi T, Kurita T, Odani T, Fujieda Y, Otomo K, et al. Hepatitis B virus reactivation by immunosuppressive therapy in patients with autoimmune diseases: risk analysis in Hepatitis B surface antigen-negative cases. J Rheumatol. 2011; 38:2209–2214. PMID: 21844146.
Article14. Charpin C, Guis S, Colson P, Borentain P, Mattéi JP, Alcaraz P, et al. Safety of TNF-blocking agents in rheumatic patients with serology suggesting past hepatitis B state: results from a cohort of 21 patients. Arthritis Res Ther. 2009; 11:R179. PMID: 19941642.
Article15. Yang BM, Kim CH, Kim JY. Cost of chronic hepatitis B infection in South Korea. J Clin Gastroenterol. 2004; 38(10 Suppl 3):S153–S157. PMID: 15602164.
Article16. Kim H, Shin AR, Chung HH, Kim MK, Lee JS, Shim JJ, et al. Recent trends in hepatitis B virus infection in the general Korean population. Korean J Intern Med. 2013; 28:413–419. PMID: 23864799.
Article17. Lee YH, Bae SC, Song GG. Hepatitis B virus (HBV) reactivation in rheumatic patients with hepatitis core antigen (HBV occult carriers) undergoing anti-tumor necrosis factor therapy. Clin Exp Rheumatol. 2013; 31:118–121.18. Mori S, Fujiyama S. Hepatitis B virus reactivation associated with antirheumatic therapy: risk and prophylaxis recommendations. World J Gastroenterol. 2015; 21:10274–10289. PMID: 26420955.
Article19. Lin CT, Huang WN, Hsieh CW, Chen YM, Chen DY, Hsieh TY, et al. Safety and effectiveness of tocilizumab in treating patients with rheumatoid arthritis - A three-year study in Taiwan. J Microbiol Immunol Infect. 2017; 7. 01. [Epub]. DOI: 10.1016/j.jmii.2017.04.002.
Article20. Papalopoulos I, Fanouriakis A, Kougkas N, Flouri I, Sourvinos G, Bertsias G, et al. Liver safety of non-tumour necrosis factor inhibitors in rheumatic patients with past hepatitis B virus infection: an observational, controlled, long-term study. Clin Exp Rheumatol. 2018; 36:102–109. PMID: 28850029.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Sepsis Caused by Cellulitis in a Patient with Rheumatoid Arthritis after Tocilizumab Treatment
- A Case of Polyarthritis Associated with Reactivation of Chronic Hepatitis B Virus Infection
- Prevention of Viral Hepatitis and Vaccination
- Description of the Efficacy and Safety of Three New Biologics in the Treatment of Rheumatoid Arthritis
- Tocilizumab-induced Transaminitis in a Seropositive Rheumatoid Arthritis Patient with Macrophage Activation Syndrome