Nutr Res Pract.
2017 Jun;11(3):190-197. 10.4162/nrp.2017.11.3.190.
Melanin extract from Gallus gallus domesticus promotes proliferation and differentiation of osteoblastic MG-63 cells via bone morphogenetic protein-2 signaling
- Affiliations
-
- 1Department of Food Science and Technology, Seoul National University of Science & Technology, Seoul 01811, Korea.
- 2Department of Advanced Materials Engineering, Daejeon University, Daejeon 34520, Korea.
- 3Department of Oriental Medicine, Daejeon University, Daejeon 34520, Korea.
- 4Division of Food Bioscience, Konkuk University, 268, Chungwon-daero, Chunju, Chungbuk 27478, Korea. anjhee@hanmail.net
- KMID: 2407766
- DOI: http://doi.org/10.4162/nrp.2017.11.3.190
Abstract
- BACKGROUND/OBJECTIVES
Gallus gallus domesticus (GD) is a natural mutant breed of chicken in Korea with an atypical characterization of melanin in its tissue. This study investigated the effects of melanin extracts of GD on osteoblast differentiation and inhibition of osteoclast formation.
MATERIALS/METHODS
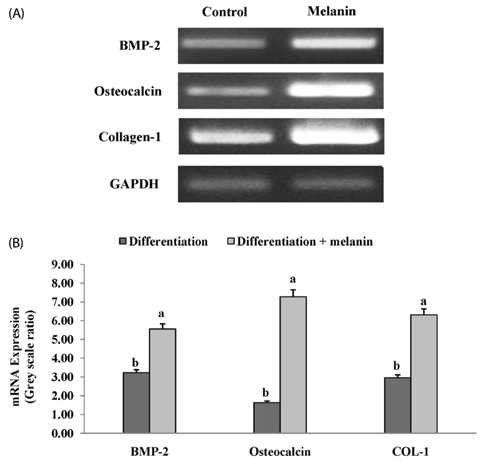
The effects of the melanin extract of GD on human osteoblast MG-63 cell differentiation were examined by evaluating cell viability, osteoblast differentiation, and expression of osteoblast-specific transcription factors such as bone morphogenetic protein 2 (BMP-2), small mothers against decapentaplegic homologs 5 (SMAD5), runt-related transcription factor 2 (RUNX2), osteocalcin and type 1 collagen (COL-1) by reverse transcription-polymerase chain reaction and western blotting analysis. We investigated the inhibitory effect of melanin on the osteoclasts formation through tartrate-resistant acid phosphatase (TRAP) activity and TRAP stains in Raw 264.7 cell.
RESULTS
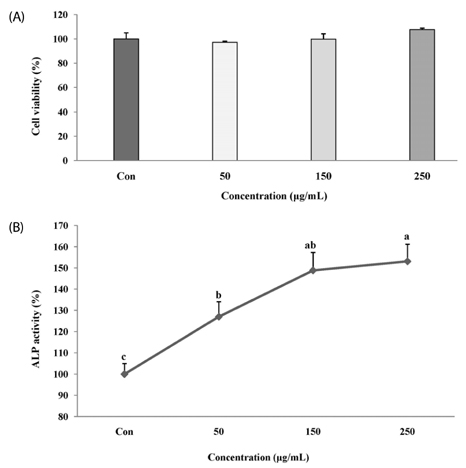
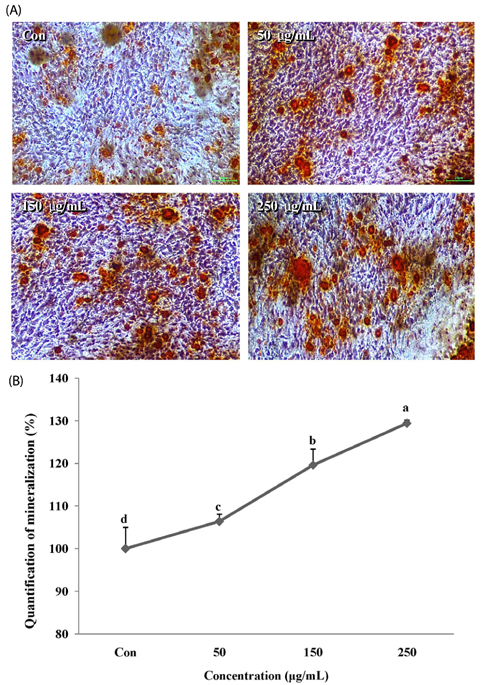
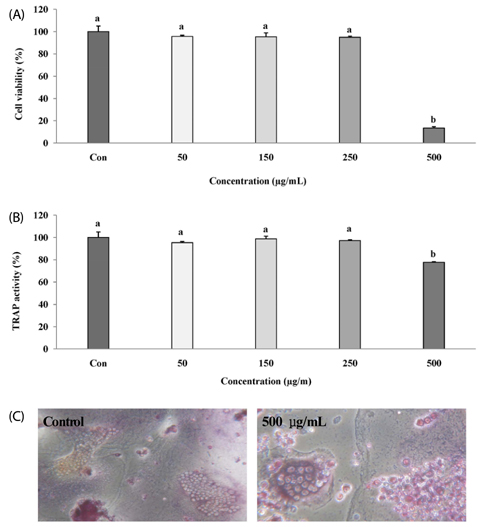
The melanin extract of GD was not cytotoxic to MG-63 cells at concentrations of 50-250 µg/mL. Alkaline phosphatase (ALP) activity and bone mineralization of melanin extract-treated cells increased in a dose-dependent manner from 50 to 250 µg/mL and were 149% and 129% at 250 µg/mL concentration, respectively (P < 0.05). The levels of BMP-2, osteocalcin, and COL-1 gene expression were significantly upregulated by 1.72-, 4.44-, and 2.12-fold in melanin-treated cells than in the control cells (P < 0.05). The levels of RUNX2 and SMAD5 proteins were higher in melanin-treated cells than in control vehicle-treated cells. The melanin extract attenuated the formation of receptor activator of nuclear factor kappa-B ligand-induced TRAP-positive multinucleated RAW 264.7 cells by 22%, and was 77% cytotoxic to RAW 264.7 macrophages at a concentration of 500 µg/mL.
CONCLUSIONS
This study provides evidence that the melanin extract promoted osteoblast differentiation by activating BMP/SMADs/RUNX2 signaling and regulating transcription of osteogenic genes such as ALP, type I collagen, and osteocalcin. These results suggest that the effective osteoblastic differentiation induced by melanin extract from GD makes it potentially useful in maintaining bone health.
MeSH Terms
-
Acid Phosphatase
Alkaline Phosphatase
Blotting, Western
Bone Morphogenetic Protein 2
Calcification, Physiologic
Cell Differentiation
Cell Survival
Chickens*
Collagen Type I
Coloring Agents
Gene Expression
Humans
Korea
Macrophages
Melanins*
Osteoblasts*
Osteocalcin
Osteoclasts
RAW 264.7 Cells
Smad Proteins
Smad5 Protein
Transcription Factors
Acid Phosphatase
Alkaline Phosphatase
Bone Morphogenetic Protein 2
Collagen Type I
Coloring Agents
Melanins
Osteocalcin
Smad Proteins
Smad5 Protein
Transcription Factors
Figure
Reference
-
1. Vondracek SF, Linnebur SA. Diagnosis and management of osteoporosis in the older senior. Clin Interv Aging. 2009; 4:121–136.
Article2. Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003; 423:337–342.
Article3. Feng J, Shi Z, Ye Z. Effects of metabolites of the lignans enterolactone and enterodiol on osteoblastic differentiation of MG-63 cells. Biol Pharm Bull. 2008; 31:1067–1070.
Article4. Valero MA, Loinaz C, Larrodera L, Leon M, Moreno E, Hawkins F. Calcitonin and bisphosphonates treatment in bone loss after liver transplantation. Calcif Tissue Int. 1995; 57:15–19.
Article5. Kardinaal AF, Morton MS, Brüggemann-Rotgans IE, van Beresteijn EC. Phyto-oestrogen excretion and rate of bone loss in postmenopausal women. Eur J Clin Nutr. 1998; 52:850–855.
Article6. Richy F, Schacht E, Bruyere O, Ethgen O, Gourlay M, Reginster JY. Vitamin D analogs versus native vitamin D in preventing bone loss and osteoporosis-related fractures: a comparative meta-analysis. Calcif Tissue Int. 2005; 76:176–186.
Article7. Alekel DL, Germain AS, Peterson CT, Hanson KB, Stewart JW, Toda T. Isoflavone-rich soy protein isolate attenuates bone loss in the lumbar spine of perimenopausal women. Am J Clin Nutr. 2000; 72:844–852.
Article8. Cho CM, Park CK, Lee MY, Lew ID. Physicochemical characteristics of silky fowl (Gallus domesticus var. silkies). Korean J Food Sci Anim Resour. 2006; 26:306–314.9. Lin LC, Chen WT. The study of antioxidant effects in melanins extracted from various tissues of animals. Asian-Australas J Anim Sci. 2005; 18:277–281.
Article10. Chae HS, An CN, Yoo YM, Park BY, Cho SH, Kim JH, Lee JM, Choi YI. Quality stability of high pressure boiled extract of ogol chicken during storage periods. Korea J Poult Sci. 2002; 29:279–286.11. Liu JH. Study on antioxidation, anti-hypertension and hematopoiesis of taihe black-bone silky fowl (Gallus gallus domesticus brission) bioactive peptides [PhD thesis]. Nanchang: Nanchang University;2011.12. Wang Y. Study on several functionalities of taihe black-bone silky fowl peptides [master's thesis]. Nanchang: Nanchang University;2011.13. Yoo HS, Chung KH, Lee KJ, Kim DH, An JH. Effect of Gallus gallus var. domesticus (Yeonsan ogolgye) extracts on osteoblast differentiation and osteoclast formation. Microbiol Biotechnol Lett. 2015; 43:322–329.
Article14. Harki E, Talou T, Dargent R. Purification, characterisation and analysis of melanin extracted from Tuber melanosporum Vitt. Food Chem. 1997; 58:69–73.
Article15. Liu SY, Shawkey MD, Parkinson D, Troy TP, Ahmed M. Elucidation of the chemical composition of avian melanin. RSC Adv. 2014; 4:40396–40399.
Article16. Sajjan S, Kulkarni G, Yaligara V, Kyoung L, Karegoudar TB. Purification and physiochemical characterization of melanin pigment from Klebsiella sp. GSK. J Microbiol Biotechnol. 2010; 20:1513–1520.
Article17. Turick CE, Tisa LS, Caccavo F Jr. Melanin production and use as a soluble electron shuttle for Fe(III) oxide reduction and as a terminal electron acceptor by Shewanella algae BrY. Appl Environ Microbiol. 2002; 68:2436–2444.
Article18. Ye M, Guo GY, Lu Y, Song S, Wang HY, Yang L. Purification, structure and anti-radiation activity of melanin from Lachnum YM404. Int J Biol Macromol. 2014; 63:170–176.
Article19. Javed A, Bae JS, Afzal F, Gutierrez S, Pratap J, Zaidi SK, Lou Y, van Wijnen AJ, Stein JL, Stein GS, Lian JB. Structural coupling of Smad and Runx2 for execution of the BMP2 osteogenic signal. J Biol Chem. 2008; 283:8412–8422.
Article20. Javed A, Afzal F, Bae JS, Gutierrez S, Zaidi K, Pratap J, van Wijnen AJ, Stein JL, Stein GS, Lian JB. Specific residues of RUNX2 are obligatory for formation of BMP2-induced RUNX2-SMAD complex to promote osteoblast differentiation. Cells Tissues Organs. 2009; 189:133–137.
Article21. de Cássia R, Pombeiro-Sponchiado SR. Antioxidant activity of the melanin pigment extracted from Aspergillus nidulans. Biol Pharm Bull. 2005; 28:1129–1131.
Article22. Xia L, Yin Z, Mao L, Wang X, Liu J, Jiang X, Zhang Z, Lin K, Chang J, Fang B. Akermanite bioceramics promote osteogenesis, angiogenesis and suppress osteoclastogenesis for osteoporotic bone regeneration. Sci Rep. 2016; 6:22005.
Article23. Kim BS, Kang HJ, Park JY, Lee J. Fucoidan promotes osteoblast differentiation via JNK- and ERK-dependent BMP2-Smad 1/5/8 signaling in human mesenchymal stem cells. Exp Mol Med. 2015; 47:e128.
Article24. Jeon EJ, Lee DH, Kim YJ, Ahn J, Kim MJ, Hwang JT, Hur J, Kim M, Jang YJ, Ha TY, Seo DH, Lee JS, Sung MJ, Jung CH. Effects of yuja peel extract and its flavanones on osteopenia in ovariectomized rats and osteoblast differentiation. Mol Nutr Food Res. 2016; 60:2587–2601.
Article25. Pan W, Quarles LD, Song LH, Yu YH, Jiao C, Tang HB, Jiang CH, Deng HW, Li YJ, Zhou HH, Xiao ZS. Genistein stimulates the osteoblastic differentiation via NO/cGMP in bone marrow culture. J Cell Biochem. 2005; 94:307–316.
Article26. Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, Sato M, Okamoto R, Kitamura Y, Yoshiki S, Kishimoto T. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997; 89:755–764.
Article27. Xiao G, Gopalakrishnan R, Jiang D, Reith E, Benson MD, Franceschi RT. Bone morphogenetic proteins, extracellular matrix, and mitogen-activated protein kinase signaling pathways are required for osteoblast-specific gene expression and differentiation in MC3T3-E1 cells. J Bone Miner Res. 2002; 17:101–110.
Article28. Suzawa M, Takeuchi Y, Fukumoto S, Kato S, Ueno N, Miyazono K, Matsumoto T, Fujita T. Extracellular matrix-associated bone morphogenetic proteins are essential for differentiation of murine osteoblastic cells in vitro. Endocrinology. 1999; 140:2125–2133.
Article29. Lee KS, Kim HJ, Li QL, Chi XZ, Ueta C, Komori T, Wozney JM, Kim EG, Choi JY, Ryoo HM, Bae SC. Runx2 is a common target of transforming growth factor beta1 and bone morphogenetic protein 2, and cooperation between Runx2 and Smad5 induces osteoblastspecific gene expression in the pluripotent mesenchymal precursor cell line C2C12. Mol Cell Biol. 2000; 20:8783–8792.
Article30. Bae JS, Gutierrez S, Narla R, Pratap J, Devados R, van Wijnen AJ, Stein JL, Stein GS, Lian JB, Javed A. Reconstitution of Runx2/Cbfa1-null cells identifies a requirement for BMP2 signaling through a Runx2 functional domain during osteoblast differentiation. J Cell Biochem. 2007; 100:434–449.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A new cestode Raillietina (Skrjabinia) doggaddaensis n. sp. from Gallus gallus domesticus (L.) from India
- Low-Intensity Pulsed Ultrasound Promotes BMP9 Induced Osteoblastic Differentiation in Rat Dedifferentiated Fat Cells
- Effects of Scytosiphon lomentaria on osteoblastic proliferation and differentiation of MC3T3-E1 cells
- Bone Morphogenetic Protein Receptor in the Osteogenic Differentiation of Rat Bone Marrow Stromal Cells
- Distribution and ontogeny of gastrin- and serotonin-immunoreactive cells in the proventriculus of developing chick, Gallus gallus domestica