Nutr Res Pract.
2017 Jun;11(3):180-189. 10.4162/nrp.2017.11.3.180.
Increased glucose metabolism and alpha-glucosidase inhibition in Cordyceps militaris water extract-treated HepG2 cells
- Affiliations
-
- 1Well-being Bioproducts RIC, Kangwon National University, Gangwon 25209, Korea. mchoe@kangwon.ac.kr
- 2National Development Institute of Korean Medicine, Gyeongbuk 38540, Korea.
- 3Department of Bio-Health Technology, Kangwon National University, 1 Gangwondaehak-gil, Chuncheon, Gangwon 24341, Korea.
- 4Department of Biochemistry, Hallym University College of Medicine, Gangwon 24252, Korea.
- KMID: 2407765
- DOI: http://doi.org/10.4162/nrp.2017.11.3.180
Abstract
- BACKGROUND/OBJECTIVES
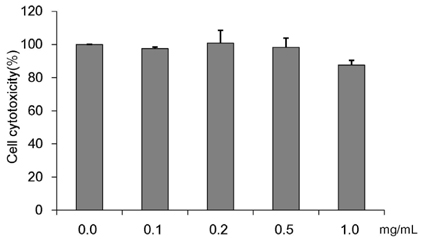
Recent living condition improvements, changes in dietary habits, and reductions in physical activity are contributing to an increase in metabolic syndrome symptoms including diabetes and obesity. Through such societal developments, humankind is continuously exposed to metabolic diseases such as diabetes, and the number of the victims is increasing. This study investigated Cordyceps militaris water extract (CMW)-induced glucose uptake in HepG2 cells and the effect of CMW treatment on glucose metabolism.
MATERIALS/METHODS
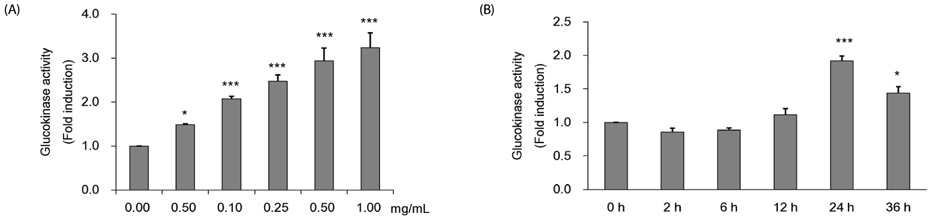
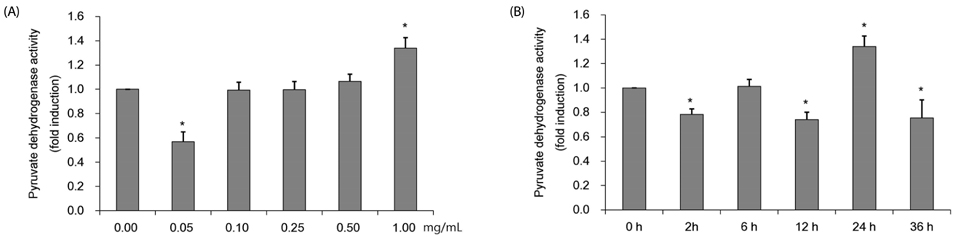
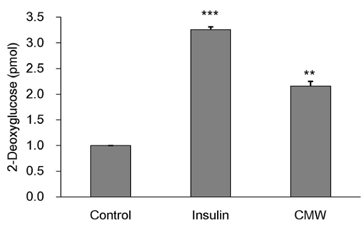
Colorimetric assay kits were used to determine the glucokinase (GK) and pyruvate dehydrogenase (PDH) activities, glucose uptake, and glycogen content. Either RT-PCR or western blot analysis was performed for quantitation of glucose transporter 2 (GLUT2), hepatocyte nuclear factor 1 alpha (HNF-1α), phosphatidylinositol 3-kinase (PI3k), protein kinase B (Akt), phosphorylated AMP-activated protein kinase (pAMPK), phosphoenolpyruvate carboxykinase, GK, PDH, and glycogen synthase kinase 3 beta (GSK-3β) expression levels. The α-glucosidase inhibitory activities of acarbose and CMW were evaluated by absorbance measurement.
RESULTS
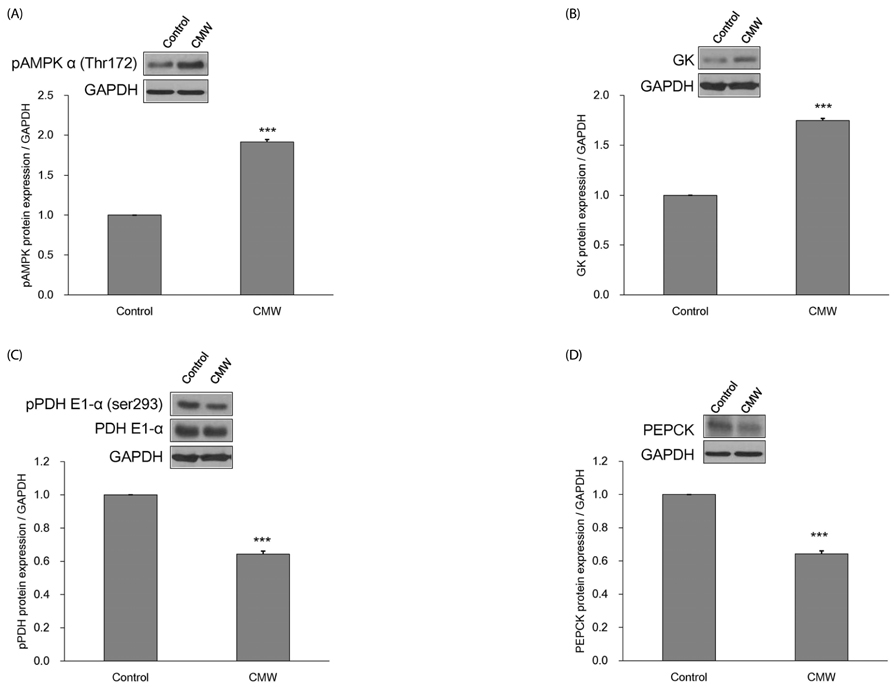
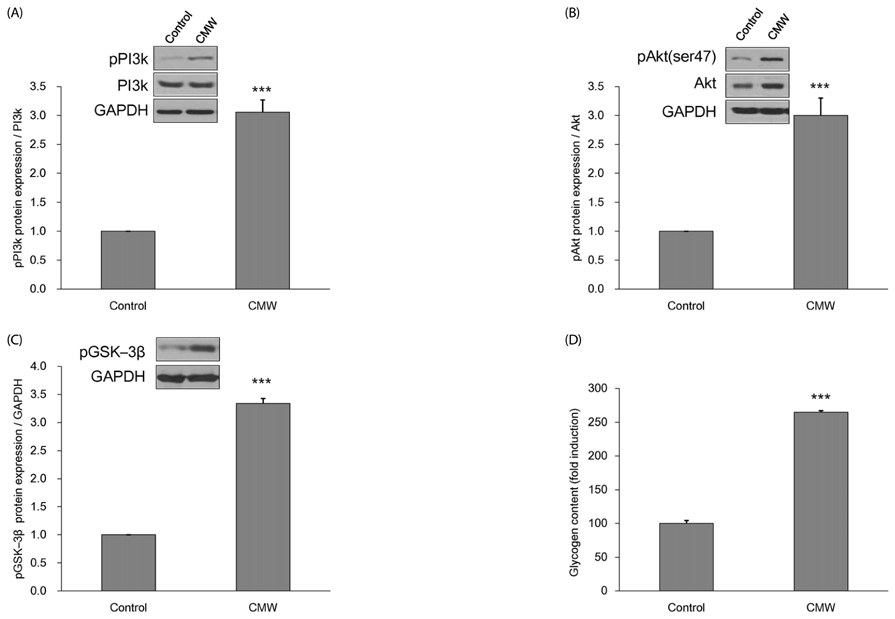
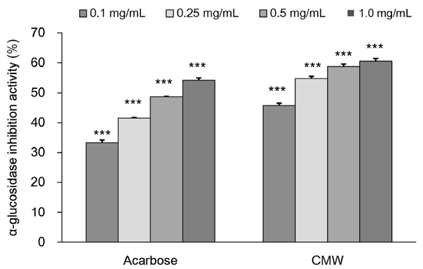
CMW induced glucose uptake in HepG2 cells by increasing GLUT2 through HNF-1α expression stimulation. Glucose in the cells increased the CMW-induced phosphorylation of AMPK. In turn, glycolysis was stimulated, and glyconeogenesis was inhibited. Furthermore, by studying the mechanism of action of PI3k, Akt, and GSK-3β, and measuring glycogen content, the study confirmed that the glucose was stored in the liver as glycogen. Finally, CMW resulted in a higher level of α-glucosidase inhibitory activity than that from acarbose.
CONCLUSION
CMW induced the uptake of glucose into HepG2 cells, as well, it induced metabolism of the absorbed glucose. It is concluded that CMW is a candidate or potential use in diabetes prevention and treatment.
MeSH Terms
-
Acarbose
alpha-Glucosidases*
AMP-Activated Protein Kinases
Blotting, Western
Cordyceps*
Food Habits
Glucokinase
Glucose Transport Proteins, Facilitative
Glucose*
Glycogen
Glycogen Synthase Kinase 3
Glycolysis
Hep G2 Cells*
Hepatocyte Nuclear Factor 1-alpha
Hypoglycemic Agents
Liver
Metabolic Diseases
Metabolism*
Motor Activity
Obesity
Oxidoreductases
Phosphatidylinositol 3-Kinase
Phosphoenolpyruvate
Phosphorylation
Proto-Oncogene Proteins c-akt
Pyruvic Acid
Social Conditions
Water*
AMP-Activated Protein Kinases
Acarbose
Glucokinase
Glucose
Glucose Transport Proteins, Facilitative
Glycogen
Glycogen Synthase Kinase 3
Hepatocyte Nuclear Factor 1-alpha
Hypoglycemic Agents
Oxidoreductases
Phosphatidylinositol 3-Kinase
Phosphoenolpyruvate
Proto-Oncogene Proteins c-akt
Pyruvic Acid
Water
alpha-Glucosidases
Figure
Reference
-
1. Rotshteyn Y, Zito SW. Application of modified in vitro screening procedure for identifying herbals possessing sulfonylurea-like activity. J Ethnopharmacol. 2004; 93:337–344.
Article2. Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011; 94:311–321.
Article3. Lee SH, Lee JK, Kim IH. Trends and perspectives in the development of antidiabetic drugs for type 2 diabetes mellitus. Korean J Microbiol Biotechnol. 2012; 40:180–185.
Article4. Sawada K, Yamashita Y, Zhang T, Nakagawa K, Ashida H. Glabridin induces glucose uptake via the AMP-activated protein kinase pathway in muscle cells. Mol Cell Endocrinol. 2014; 393:99–108.
Article5. Yuan HD, Kim SJ, Quan HY, Huang H, Chung SH. Ginseng leaf extract prevents high fat diet-induced hyperglycemia and hyperlipidemia through AMPK activation. J Ginseng Res. 2010; 34:369–375.
Article6. Cory JG, Suhadolnik RJ, Resnick B, Rich MA. Incorporation of cordycepin (3′-deoxyadenosine) into ribonucleic acid and deoxyribonucleic acid of human tumor cells. Biochim Biophys Acta. 1965; 103:646–653.
Article7. Kwon YM, Cho SM, Kim JH, Lee JH, Lee YA, Lee SJ, Lee MW. Hypoglycemic effects of Cordyceps militaris. Korean J Pharmacogn. 2001; 32:327–329.8. Koh JB, Choi MA. Effect of Cordyceps militaris on lipid metabolism in rats fed cholesterol diet. Korean J Nutr. 2001; 34:265–270.9. Jo WS, Nam BH, Oh SJ, Choi YJ, Kang EY, Hong SH, Lee SH, Jeong MH. Hepatic protective effect and single-dose toxicity study of water extract of Cordyceps militaris grown upon protaetia dreujtarsis. Korean J Food Sci Technol. 2008; 40:106–110.10. Kim HS, Roh YJ, Choe M. Cordyceps militaris increases hepatic glucokinase activities. J Korean Soc Food Sci Nutr. 2005; 34:158–161.11. Kiho T, Hui J, Yamane A, Ukai S. Polysaccharides in fungi. XXXII. Hypoglycemic activity and chemical properties of a polysaccharide from the cultural mycelium of Cordyceps sinensis. Biol Pharm Bull. 1993; 16:1291–1293.
Article12. Choe M, Kim DJ, Lee HJ, You JK, Seo DJ, Lee JH, Chung MJ. A study on the glucose-regulating enzymes and antioxidant activities of water extracts from medicinal herbs. J Korean Soc Food Sci Nutr. 2008; 37:542–547.
Article13. Moore MC, Coate KC, Winnick JJ, An Z, Cherrington AD. Regulation of hepatic glucose uptake and storage in vivo. Adv Nutr. 2012; 3:286–294.
Article14. Cherrington AD. Banting Lecture 1997. Control of glucose uptake and release by the liver in vivo. Diabetes. 1999; 48:1198–1214.
Article15. Mueckler M, Thorens B. The SLC2 (GLUT) family of membrane transporters. Mol Aspects Med. 2013; 34:121–138.
Article16. Shimizu T, Parker JC, Najafi H, Matschinsky FM. Control of glucose metabolism in pancreatic β-cells by glucokinase, hexokinase and phosphofructokinase; model study with cell lines derived from β-cells. Diabetes. 1988; 37:1524–1530.
Article17. Matschinsky FM. Glucokinase as glucose sensor and metabolic signal generator in pancreatic β-cells and hepatocytes. Diabetes. 1990; 39:647–652.
Article18. Seoane J, Gómez-Foix AM, O'Doherty RM, Gómez-Ara C, Newgard CB, Guinovart JJ. Glucose 6-phosphate produced by glucokinase, but not hexokinase I, promotes the activation of hepatic glycogen synthase. J Biol Chem. 1996; 271:23756–23760.
Article19. Cordero-Herrera I, Martín MA, Bravo L, Goya L, Ramos S. Cocoa flavonoids improve insulin signalling and modulate glucose production via AKT and AMPK in HepG2 cells. Mol Nutr Food Res. 2013; 57:974–985.
Article20. Nordlie RC, Foster JD, Lange AJ. Regulation of glucose production by the liver. Annu Rev Nutr. 1999; 19:379–406.
Article21. Cerf ME. High fat diet modulation of glucose sensing in the beta-cell. Med Sci Monit. 2007; 13:RA12–RA17.22. Corton JM, Gillespie JG, Hardie DG. Role of the AMP-activated protein kinase in the cellular stress response. Curr Biol. 1994; 4:315–324.
Article23. Winder WW, Thomson DM. Cellular energy sensing and signaling by AMP-activated protein kinase. Cell Biochem Biophys. 2007; 47:332–347.
Article24. Zhang BB, Zhou G, Li C. AMPK: an emerging drug target for diabetes and the metabolic syndrome. Cell Metab. 2009; 9:407–416.
Article25. Lee ES, Uhm KO, Lee YM, Han M, Lee M, Park JM, Suh PG, Park SH, Kim HS. CAPE (caffeic acid phenethyl ester) stimulates glucose uptake through AMPK (AMP-activated protein kinase) activation in skeletal muscle cells. Biochem Biophys Res Commun. 2007; 361:854–858.
Article26. Lin CL, Lin JK. Epigallocatechin gallate (EGCG) attenuates high glucose-induced insulin signaling blockade in human HepG2 hematoma cells. Mol Nutr Food Res. 2008; 52:930–939.
Article27. Ha T, Trung TN, Hien TT, Dao TT, Yim N, Ngoc TM, Oh WK, Bae K. Selected compounds derived from Moutan Cortex stimulated glucose uptake and glycogen synthesis via AMPK activation in human HepG2 cells. J Ethnopharmacol. 2010; 131:417–424.
Article28. Whiteman EL, Cho H, Birnbaum MJ. Role of Akt/protein kinase B in metabolism. Trends Endocrinol Metab. 2002; 13:444–451.
Article29. Brunet A, Datta SR, Greenberg ME. Transcription-dependent and -independent control of neuronal survival by the PI3K-Akt signaling pathway. Curr Opin Neurobiol. 2001; 11:297–305.
Article30. Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999; 13:2905–2927.
Article31. Brazil DP, Hemmings BA. Ten years of protein kinase B signalling: a hard Akt to follow. Trends Biochem Sci. 2001; 26:657–664.
Article32. Kumar S, Narwal S, Kumar V, Prakash O. α-glucosidase inhibitors from plants: a natural approach to treat diabetes. Pharmacogn Rev. 2011; 5:19–29.
Article33. Chang X, Li J, Xie X, Huang K, Huag H. Effect of TangMaijiaoTai on regulating the glycolipid metabolism and the production of glucokinase and low density lipoprotein receptor protein in diabetic rats with hyperlipidemia. Chin Pharmacol Bull. 2013; 29:234–237.34. Ferriero R, Brunetti-Pierri N. Phenylbutyrate increases activity of pyruvate dehydrogenase complex. Oncotarget. 2013; 4:804–805.
Article35. Arjunan P, Nemeria N, Brunskill A, Chandrasekhar K, Sax M, Yan Y, Jordan F, Guest JR, Furey W. Structure of the pyruvate dehydrogenase multienzyme complex E1 component from Escherichia coli at 1.85 A resolution. Biochemistry. 2002; 41:5213–5221.
Article36. Johnson JH, Newgard CB, Milburn JL, Lodish HF, Thorens B. The high Km glucose transporter of islets of Langerhans is functionally similar to the low affinity transporter of liver and has an identical primary sequence. J Biol Chem. 1990; 265:6548–6551.
Article37. Cha JY, Kim H, Kim KS, Hur MW, Ahn Y. Identification of transacting factors responsible for the tissue-specific expression of human glucose transporter type 2 isoform gene. Cooperative role of hepatocyte nuclear factors 1alpha and 3beta. J Biol Chem. 2000; 275:18358–18365.
Article38. Kang YH, Lee YS, Kim KK, Kim DJ, Kim TW, Choe M. Study on antioxidative, antidiabetic and antiobesity activity of solvent fractions of Smilax china L. leaf extract. J Nutr Health. 2013; 46:401–409.
Article39. Narasimhan A, Chinnaiyan M, Karundevi B. Ferulic acid regulates hepatic GLUT2 gene expression in high fat and fructose-induced type-2 diabetic adult male rat. Eur J Pharmacol. 2015; 761:391–397.
Article40. Im SS, Kang SY, Kim SY, Kim HI, Kim JW, Kim KS, Ahn YH. Glucose-stimulated upregulation of GLUT2 gene is mediated by sterol response element-binding protein-1c in the hepatocytes. Diabetes. 2005; 54:1684–1691.
Article41. Matsui C, Shoji I, Kaneda S, Sianipar IR, Deng L, Hotta H. Hepatitis C virus infection suppresses GLUT2 gene expression via downregulation of hepatocyte nuclear factor 1α. J Virol. 2012; 86:12903–12911.
Article42. Hardie DG. Minireview: the AMP-activated protein kinase cascade: the key sensor of cellular energy status. Endocrinology. 2003; 144:5179–5183.
Article43. Carling D. The AMP-activated protein kinase cascade--a unifying system for energy control. Trends Biochem Sci. 2004; 29:18–24.
Article44. Cool B, Zinker B, Chiou W, Kifle L, Cao N, Perham M, Dickinson R, Adler A, Gagne G, Iyengar R, Zhao G, Marsh K, Kym P, Jung P, Camp HS, Frevert E. Identification and characterization of a small molecule AMPK activator that treats key components of type 2 diabetes and the metabolic syndrome. Cell Metab. 2006; 3:403–416.
Article45. Phielix E, Szendroedi J, Roden M. The role of metformin and thiazolidinediones in the regulation of hepatic glucose metabolism and its clinical impact. Trends Pharmacol Sci. 2011; 32:607–616.
Article46. Towler MC, Hardie DG. AMP-activated protein kinase in metabolic control and insulin signaling. Circ Res. 2007; 100:328–341.
Article47. The glycolytic pathway is tightly controlled. In : Berg JM, Tymoczko JL, Stryer L, editors. Biochemistry. 5th ed. New York (NY): W H Freeman;2002. p. 668–676.48. Holness MJ, Sugden MC. Regulation of pyruvate dehydrogenase complex activity by reversible phosphorylation. Biochem Soc Trans. 2003; 31:1143–1151.
Article49. Cordero-Herrera I, Martín MÁ, Goya L, Ramos S. Cocoa flavonoids attenuate high glucose-induced insulin signalling blockade and modulate glucose uptake and production in human HepG2 cells. Food Chem Toxicol. 2014; 64:10–19.
Article50. Waltner-Law ME, Wang XL, Law BK, Hall RK, Nawano M, Granner DK. Epigallocatechin gallate, a constituent of green tea, represses hepatic glucose production. J Biol Chem. 2002; 277:34933–34940.
Article51. Kim T, Davis J, Zhang AJ, He X, Mathews ST. Curcumin activates AMPK and suppresses gluconeogenic gene expression in hepatoma cells. Biochem Biophys Res Commun. 2009; 388:377–382.
Article52. Lee J, Kim MS. The role of GSK3 in glucose homeostasis and the development of insulin resistance. Diabetes Res Clin Pract. 2007; 77:Suppl 1. S49–S57.
Article53. Hagiwara A, Cornu M, Cybulski N, Polak P, Betz C, Trapani F, Terracciano L, Heim MH, Rüegg MA, Hall MN. Hepatic mTORC2 activates glycolysis and lipogenesis through Akt, glucokinase, and SREBP1c. Cell Metab. 2012; 15:725–738.
Article54. Hao J, Chen C, Huang K, Huang J, Li J, Liu P, Huang H. Polydatin improves glucose and lipid metabolism in experimental diabetes through activating the Akt signaling pathway. Eur J Pharmacol. 2014; 745:152–165.
Article55. Mertes G. Efficacy and safety of acarbose in the treatment of type 2 diabetes: data from a 2-year surveillance study. Diabetes Res Clin Pract. 1998; 40:63–70.
Article56. Rosak C, Mertes G. Effects of acarbose on proinsulin and insulin secretion and their potential significance for the intermediary metabolism and cardiovascular system. Curr Diabetes Rev. 2009; 5:157–164.
Article57. Ahn H, Chung L, Choe E. In vitro antioxidant activity and α-glucosidase and pancreatic lipase inhibitory activities of several Korean sanchae. Korean J Food Sci Technol. 2015; 47:164–169.
Article58. Choi JH, Park YH, Lee SG, Lee SH, Yu MH, Lee MS, Park SH, Lee IS, Kim HJ. Antioxidant activities and α-glucosidase inhibition effects of chicories grown in hydroponics added with Cr3+ or selenium. J Food Hyg Saf. 2014; 29:53–59.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Anti-inflammatory Effects of Water Extract from Cordyceps militaris in Murine Macrophage
- Morphological Characteristics of Conidiogenesis in Cordyceps militaris
- Cordyceps militaris alleviates non-alcoholic fatty liver disease in ob/ob mice
- Antioxidant and antidiabetic activities of extracts from Cirsium japonicum roots
- Study of the mechanisms underlying increased glucose absorption in Smilax china L. leaf extract-treated HepG2 cells