J Vet Sci.
2018 Mar;19(2):301-308. 10.4142/jvs.2018.19.2.301.
Cytological endometritis in dairy cows: diagnostic threshold, risk factors, and impact on reproductive performance
- Affiliations
-
- 1Veterinary Medical Center and College of Veterinary Medicine, Chungbuk National University, Cheongju 28644, Korea. illhwa@cbu.ac.kr
- 2National Institute of Animal Science, Rural Development Administration, Cheonan 31000, Korea.
- KMID: 2407630
- DOI: http://doi.org/10.4142/jvs.2018.19.2.301
Abstract
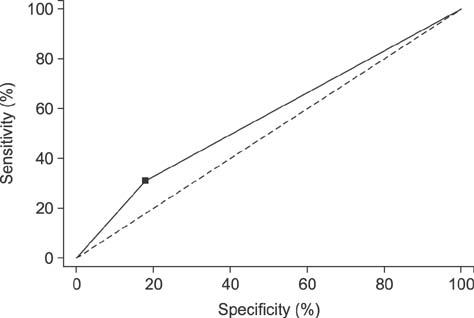
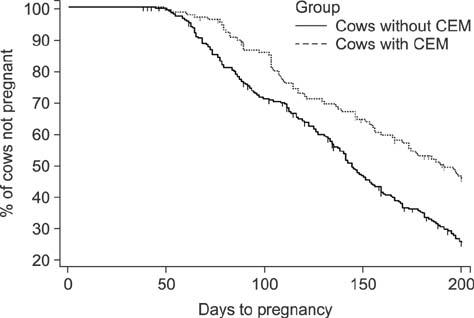
- We determined the threshold proportion of polymorphonuclear leukocytes (PMNs) for a diagnosis of cytological endometritis (CEM), the risk factors for this condition, and its impact on reproductive performance in dairy cows. Uterine cytology was performed on 407 Holstein cows 4 weeks postpartum to determine the proportions of endometrial cells and PMNs. A receiver operator characteristics curve was used to determine the threshold above which the PMN proportion affected the likelihood of cows conceiving by 200 days postpartum. The optimal threshold was ≥ 14% PMN (sensitivity, 31.3%; specificity, 81.7%; p < 0.05). The farm identity, retained placenta (odds ratio [OR] = 1.87), and septicemic metritis (OR = 3.07) were risk factors for CEM (p < 0.05). Cows with CEM were less likely to resume cyclicity (OR = 0.58) and to conceive by 200 days postpartum (hazard ratio = 0.58). Cows with CEM tended (p < 0.1) to be less likely to become pregnant after their first insemination (OR = 0.65) and to require a greater number of inseminations per conception (2.3 vs. 2.2). In conclusion, a PMN threshold of 14% defined the presence of CEM at 4 weeks postpartum. The farm, retained placenta, and septicemic metritis were risk factors for CEM, which reduces subsequent reproductive performance.
Keyword
MeSH Terms
Figure
Reference
-
1. Ahmadi MR, Kadivar A, Vatankhah M. Evaluation of polymorphonuclear (PMN) cells in cervical sample as a diagnostic technique for detection of subclinical endometritis in dairy cattle. Asian Pac J Reprod. 2016; 5:340–344.
Article2. Barlund CS, Carruthers TD, Waldner CL, Palmer CW. A comparison of diagnostic techniques for postpartum endometritis in dairy cattle. Theriogenology. 2008; 69:714–723.
Article3. Bartlett PC, Kirk JH, Wilke MA, Kaneene JB, Mather EC. Metritis complex in Michigan Holstein-Friesian cattle: incidence, descriptive epidemiology and estimated economic impact. Prev Vet Med. 1986; 4:235–248.
Article4. Bondurant RH. Inflammation in the bovine female reproductive tract. J Anim Sci. 1999; 77:Suppl 2. 101–110.
Article5. Bonnett BN, Martin SW, Gannon VP, Miller RB, Etherington WG. Endometrial biopsy in Holstein-Friesian dairy cows. III. Bacteriological analysis and correlations with histological findings. Can J Vet Res. 1991; 55:168–173.6. Bruun J, Ersbøll AK, Alban L. Risk factors for metritis in Danish dairy cows. Prev Vet Med. 2002; 54:179–190.
Article7. Cheong SH, Nydam DV, Galvão KN, Crosier BM, Gilbert RO. Cow-level and herd-level risk factors for subclinical endometritis in lactating Holstein cows. J Dairy Sci. 2011; 94:762–770.
Article8. De Rensis F, Lopez-Gatius F, García-Ispierto I, Morini G, Scaramuzzi RJ. Causes of declining fertility in dairy cows during the warm season. Theriogenology. 2017; 91:145–153.
Article9. de Vries MJ, van der Beek S, Kaal-Lansbergen LM, Ouweltjes W, Wilmink JB. Modeling of energy balance in early lactation and the effect of energy deficits in early lactation on first detected estrus postpartum in dairy cows. J Dairy Sci. 1999; 82:1927–1934.
Article10. Dubuc J, Duffield TF, Leslie KE, Walton JS, LeBlanc SJ. Definitions and diagnosis of postpartum endometritis in dairy cows. J Dairy Sci. 2010; 93:5225–5233.
Article11. Dubuc J, Duffield TF, Leslie KE, Walton JS, LeBlanc SJ. Risk factors for postpartum uterine diseases in dairy cows. J Dairy Sci. 2010; 93:5764–5771.
Article12. Gautam G, Nakao T, Yusuf M, Koike K. Prevalence of endometritis during the postpartum period and its impact on subsequent reproductive performance in two Japanese dairy herds. Anim Reprod Sci. 2009; 116:175–187.
Article13. Gilbert RO, Shin ST, Guard CL, Erb HN, Frajblat M. Prevalence of endometritis and its effects on reproductive performance of dairy cows. Theriogenology. 2005; 64:1879–1888.
Article14. Giuliodori MJ, Magnasco RP, Becu-Villalobos D, Lacau-Mengido IM, Risco CA, de la Sota RL. Clinical endometritis in an Argentinean herd of dairy cows: risk factors and reproductive efficiency. J Dairy Sci. 2013; 96:210–218.
Article15. Gröhn YT, Rajala-Schultz PJ. Epidemiology of reproductive performance in dairy cows. Anim Reprod Sci. 2000; 60-61:605–614.
Article16. Grosche A, Fürll M, Wittek T. Peritoneal fluid analysis in dairy cows with left displaced abomasum and abomasal volvulus. Vet Rec. 2012; 170:413.
Article17. Han YK, Kim IH. Risk factors for retained placenta and the effect of retained placenta on the occurrence of postpartum diseases and subsequent reproductive performance in dairy cows. J Vet Sci. 2005; 6:53–59.
Article18. Herath S, Williams EJ, Lilly ST, Gilbert RO, Dobson H, Bryant CE, Sheldon IM. Ovarian follicular cells have innate immune capabilities that modulate their endocrine function. Reproduction. 2007; 134:683–693.
Article19. Karsch FJ, Battaglia DF, Breen KM, Debus N, Harris TG. Mechanisms for ovarian cycle disruption by immune/inflammatory stress. Stress. 2002; 5:101–112.
Article20. Kasimanickam R, Duffield TF, Foster RA, Gartley CJ, Leslie KE, Walton JS, Johnson WH. A comparison of the cytobrush and uterine lavage techniques to evaluate endometrial cytology in clinically normal postpartum dairy cows. Can Vet J. 2005; 46:255–259.21. Kasimanickam R, Duffield TF, Foster RA, Gartley CJ, Leslie KE, Walton JS, Johnson WH. Endometrial cytology and ultrasonography for the detection of subclinical endometritis in postpartum dairy cows. Theriogenology. 2004; 62:9–23.
Article22. Kim IH, Kang HG. Risk factors for postpartum endometritis and the effect of endometritis on reproductive performance in dairy cows in Korea. J Reprod Dev. 2003; 49:485–491.
Article23. Kossaibati MA, Esslemont RJ. The costs of production diseases in dairy herds in England. Vet J. 1997; 154:41–51.
Article24. LeBlanc SJ, Duffield TF, Leslie KE, Bateman KG, Keefe GP, Walton JS, Johnson WH. Defining and diagnosing postpartum clinical endometritis and its impact on reproductive performance in dairy cows. J Dairy Sci. 2002; 85:2223–2236.
Article25. Leslie KE. The events of normal and abnormal postpartum reproductive endocrinology and uterine involution in dairy cows: a review. Can Vet J. 1983; 24:67–71.26. Loeffler SH, de Vries MJ, Schukken YH. The effects of time of disease occurrence, milk yield, and body condition on fertility of dairy cows. J Dairy Sci. 1999; 82:2589–2604.
Article27. Lombard JE, Garry FB, Tomlinson SM, Garber LP. Impacts of dystocia on health and survival of dairy calves. J Dairy Sci. 2007; 90:1751–1760.
Article28. McDougall S, Macaulay R, Compton C. Association between endometritis diagnosis using a novel intravaginal device and reproductive performance in dairy cattle. Anim Reprod Sci. 2007; 99:9–23.
Article29. Prunner I, Wagener K, Pothmann H, Ehling-Schulz M, Drillich M. Risk factors for uterine diseases on small- and medium-sized dairy farms determined by clinical, bacteriological, and cytological examinations. Theriogenology. 2014; 82:857–865.
Article30. Pursley JR, Mee MO, Wiltbank MC. Synchronization of ovulation in dairy cows using PGF2α and GnRH. Theriogenology. 1995; 44:915–923.
Article31. Santos JE, Rutigliano HM, Sá Filho MF. Risk factors for resumption of postpartum estrous cycles and embryonic survival in lactating dairy cows. Anim Reprod Sci. 2009; 110:207–221.
Article32. Sheldon IM, Cronin J, Goetze L, Donofrio G, Schuberth HJ. Defining postpartum uterine disease and the mechanisms of infection and immunity in the female reproductive tract in cattle. Biol Reprod. 2009; 81:1025–1032.
Article33. Sheldon IM, Lewis GS, LeBlanc S, Gilbert RO. Defining postpartum uterine disease in cattle. Theriogenology. 2006; 65:1516–1530.
Article34. Sheldon IM, Noakes DE, Rycroft AN, Pfeiffer DU, Dobson H. Influence of uterine bacterial contamination after parturition on ovarian dominant follicle selection and follicle growth and function in cattle. Reproduction. 2002; 123:837–845.
Article35. Studer E, Morrow DA. Postpartum evaluation of bovine reproductive potential: comparison of findings from genital tract examination per rectum, uterine culture, and endometrial biopsy. J Am Vet Med Assoc. 1978; 172:489–494.36. Wagener K, Gabler C, Drillich M. A review of the ongoing discussion about definition, diagnosis and pathomechanism of subclinical endometritis in dairy cows. Theriogenology. 2017; 94:21–30.
Article37. Walsh RB, Kelton DF, Duffield TF, Leslie KE, Walton JS, LeBlanc SJ. Prevalence and risk factors for postpartum anovulatory condition in dairy cows. J Dairy Sci. 2007; 90:315–324.
Article38. Westermann S, Drillich M, Kaufmann TB, Madoz LV, Heuwieser W. A clinical approach to determine false positive findings of clinical endometritis by vaginoscopy by the use of uterine bacteriology and cytology in dairy cows. Theriogenology. 2010; 74:1248–1255.
Article39. Williams EJ, Fischer DP, Pfeiffer DU, England GC, Noakes DE, Dobson H, Sheldon IM. Clinical evaluation of postpartum vaginal mucus reflects uterine bacterial infection and the immune response in cattle. Theriogenology. 2005; 63:102–117.
Article40. Zain AED, Nakao T, Raouf MA, Moriyoshi M, Kawata K, Moritsu Y. Factors in the resumption of ovarian activity and uterine involution in postpartum dairy cows. Anim Reprod Sci. 1995; 38:203–214.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Risk factors for repeat breeder dairy cows and their impacts on reproductive performance
- Pregnancy loss in dairy cows: the contributing factors, the effects on reproductive performance and the economic impact
- Risk factors for retained placenta and the effect of retained placenta on the occurrence of postpartum diseases and subsequent reproductive performance in dairy cows
- Advancing parity is associated with high milk production at the cost of body condition and increased periparturient disorders in dairy herds
- The impact of the duration of retained placenta on postpartum diseases and culling rates in dairy cows