J Vet Sci.
2018 Mar;19(2):260-270. 10.4142/jvs.2018.19.2.260.
A two-component signal transduction system contributes to the virulence of Riemerella anatipestifer
- Affiliations
-
- 1Key Lab of Animal Bacteriology, Ministry of Agriculture, Nanjing Agricultural University, Nanjing 210095, China. vszw@njau.edu.cn
- KMID: 2407625
- DOI: http://doi.org/10.4142/jvs.2018.19.2.260
Abstract
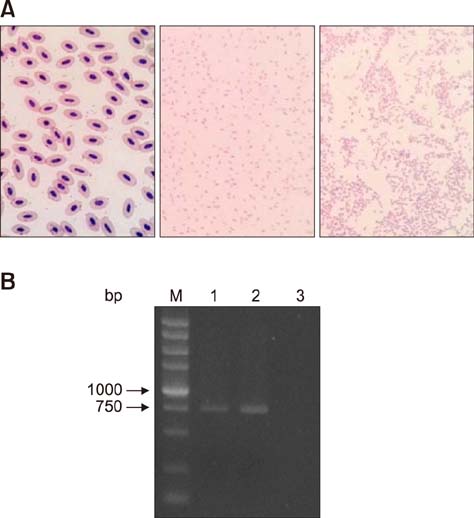
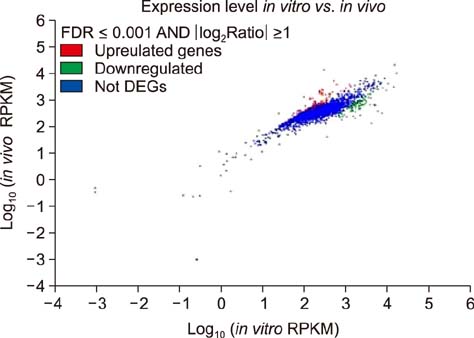
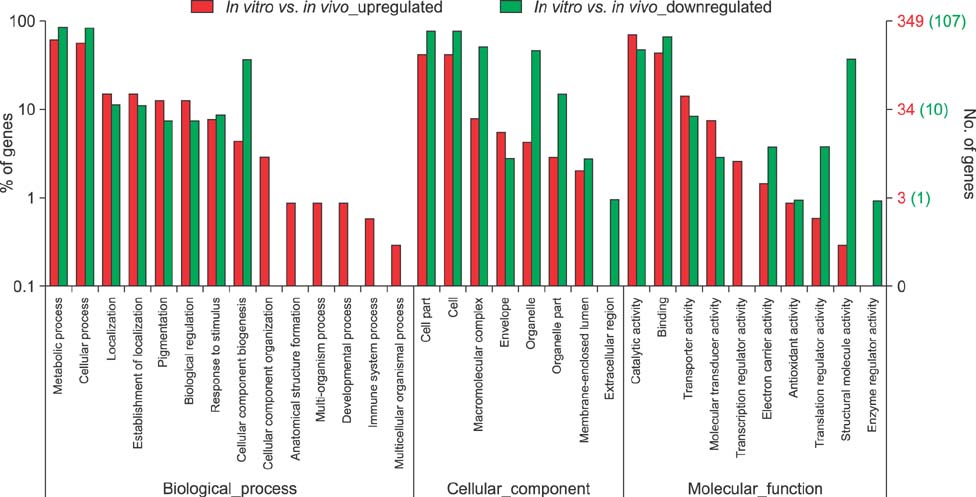
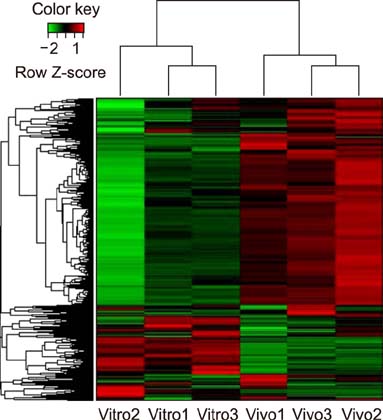
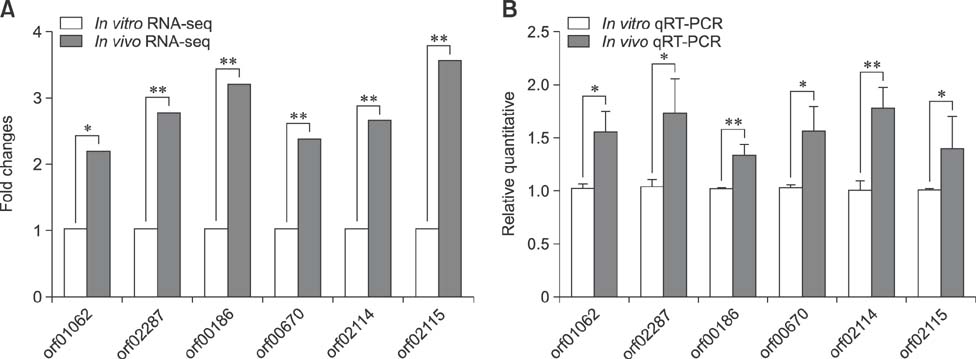
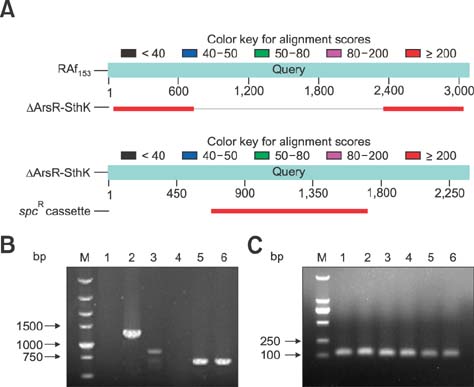
- Similar to other studies of bacterial pathogens, current studies of the pathogenesis of Riemerella anatipestifer (RA) are focused mainly on in vitro culture conditions. To elucidate further the pathogenesis of RA in vivo, bacterial RNA was extracted from overnight tryptic soy broth cultures (in vitro) and from the blood of infected ducks (in vivo) for comparative RNA sequencing analysis. In total, 682 upregulated genes were identified in vivo. Among the upregulated genes, a signal transduction response regulator (ArsR) and a signal transduction histidine kinase (SthK) were predicted to be located on the same operon. A mutant was constructed by deletion of both of these genes. Duck infection tests showed that genes ArsR and SthK were related to the virulence of the pathogen in vivo. Differentially expressed genes identified by comparison of in vitro and in vivo conditions provided an insight into the physiological process of RA infection and provided an opportunity to identify additional virulence factors.
MeSH Terms
Figure
Reference
-
1. Agari Y, Kashihara A, Yokoyama S, Kuramitsu S, Shinkai A. Global gene expression mediated by Thermus thermophilus SdrP, a CRP/FNR family transcriptional regulator. Mol Microbiol. 2008; 70:60–75.
Article2. Bhattacharjee B, Simon RM, Gangadharaiah C, Karunakar P. Chemogenomics profiling of drug targets of peptidoglycan biosynthesis pathway in Leptospira interrogans by virtual screening approaches. J Microbiol Biotechnol. 2013; 23:779–784.
Article3. Calloni G, Chen T, Schermann SM, Chang HC, Genevaux P, Agostini F, Tartaglia GG, Hayer-Hartl M, Hartl FU. DnaK functions as a central hub in the E. coli chaperone network. Cell Rep. 2012; 1:251–264.4. Crasta KC, Chua KL, Subramaniam S, Frey J, Loh H, Tan HM. Identification and characterization of CAMP cohemolysin as a potential virulence factor of Riemerella anatipestifer. J Bacteriol. 2002; 184:1932–1939.
Article5. Deslandes V, Denicourt M, Girard C, Harel J, Nash JH, Jacques M. Transcriptional profiling of Actinobacillus pleuropneumoniae during the acute phase of a natural infection in pigs. BMC Genomics. 2010; 11:98.6. Farkas MH, Au ED, Sousa ME, Pierce EA. RNA-Seq: improving our understanding of retinal biology and disease. Cold Spring Harb Perspect Med. 2015; 5:a017152.
Article7. Fazli M, O'Connell A, Nilsson M, Niehaus K, Dow JM, Givskov M, Ryan RP, Tolker-Nielsen T. The CRP/FNR family protein Bcam1349 is a c-di-GMP effector that regulates biofilm formation in the respiratory pathogen Burkholderia cenocepacia. Mol Microbiol. 2011; 82:327–341.
Article8. Frota CC, Papavinasasundaram KG, Davis EO, Colston MJ. The AraC family transcriptional regulator Rv1931c plays a role in the virulence of Mycobacterium tuberculosis. Infect Immun. 2004; 72:5483–5486.
Article9. Gallegos MT, Schleif R, Bairoch A, Hofmann K, Ramos JL. Arac/XylS family of transcriptional regulators. Microbiol Mol Biol Rev. 1997; 61:393–410.
Article10. Gao Y, Liu C, Ding Y, Sun C, Zhang R, Xian M, Zhao G. Development of genetically stable Escherichia coli strains for poly(3-hydroxypropionate) production. PLoS One. 2014; 9:e97845.11. Han X, Hu Q, Ding S, Chen W, Ding C, He L, Wang X, Ding J, Yu S. Identification and immunological characteristics of chaperonin GroEL in Riemerella anatipestifer. Appl Microbiol Biotechnol. 2012; 93:1197–1205.
Article12. Hill CE, Metcalf DS, MacInnes JI. A search for virulence genes of Haemophilus parasuis using differential display RT-PCR. Vet Microbiol. 2003; 96:189–202.
Article13. Hollenstein K, Dawson RJ, Locher KP. Structure and mechanism of ABC transporter proteins. Curr Opin Struct Biol. 2007; 17:412–418.
Article14. Hu Q, Han X, Zhou X, Ding C, Zhu Y, Yu S. OmpA is a virulence factor of Riemerella anatipestifer. Vet Microbiol. 2011; 150:278–283.15. Kardos G, Nagy J, Antal M, Bistyák A, Tenk M, Kiss I. Development of a novel PCR assay specific for Riemerella anatipestifer. Lett Appl Microbiol. 2007; 44:145–148.
Article16. Khajanchi BK, Kozlova EV, Sha J, Popov VL, Chopra AK. The two-component QseBC signalling system regulates in vitro and in vivo virulence of Aeromonas hydrophila. Microbiology. 2012; 158:259–271.
Article17. Lestrate P, Delrue RM, Danese I, Didembourg C, Taminiau B, Mertens P, De Bolle X, Tibor A, Tang CM, Letesson JJ. Identification and characterization of in vivo attenuated mutants of Brucella melitensis. Mol Microbiol. 2000; 38:543–551.
Article18. Lévesque CM, Mair RW, Perry JA, Lau PC, Li YH, Cvitkovitch DG. Systemic inactivation and phenotypic characterization of two-component systems in expression of Streptococcus mutans virulence properties. Lett Appl Microbiol. 2007; 45:398–404.
Article19. Li P, Deng WQ, Li TH, Song B, Shen YH. Illumina-based de novo transcriptome sequencing and analysis of Amanita exitialis basidiocarps. Gene. 2013; 532:63–71.
Article20. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001; 25:402–408.
Article21. Lu F, Miao S, Tu J, Ni X, Xing L, Yu H, Pan L, Hu Q. The role of TonB-dependent receptor TbdR1 in Riemerella anatipestifer in iron acquisition and virulence. Vet Microbiol. 2013; 167:713–718.
Article22. Moussatova A, Kandt C, O'Mara ML, Tieleman DP. ATP-binding cassette transporters in Escherichia coli. Biochim Biophys Acta. 2008; 1778:1757–1771.23. Pierce RL, Vorhies MW. Pasteurella anatipestifer infection in Geese. Avian Dis. 1973; 17:868–870.24. Reed LJ, Muench H. A simple method of estimating fifty percent endpoints. Am J Hyg. 1938; 27:493–497.25. Riboulet-Bisson E, Le Jeune A, Benachour A, Auffray Y, Hartke A, Giard JC. Ers a Crp/Fnr-like transcriptional regulator of Enterococcus faecalis. Int J Food Microbiol. 2009; 131:71–74.26. Rutherford JC. The emerging role of urease as a general microbial virulence factor. PLoS Pathog. 2014; 10:e1004062.
Article27. Sampson TR, Saroj SD, Llewellyn AC, Tzeng YL, Weiss DS. A CRISPR/Cas system mediates bacterial innate immune evasion and virulence. Nature. 2013; 497:254–257.
Article28. Shelver D, Rajagopal L, Harris TO, Rubens CE. MtaR, a regulator of methionine transport, is critical for survival of group B streptococcus in vivo. J Bacteriol. 2003; 185:6592–6599.
Article29. Subramaniam S, Chua KL, Tan HM, Loh H, Kuhnert P, Frey J. Phylogenetic position of Riemerella anatipestifer based on 16S rRNA gene sequences. Int J Syst Bacteriol. 1997; 47:562–565.
Article30. Tan J, Lee BD, Polo-Parada L, Sengupta S. Kinetically limited differential centrifugation as an inexpensive and readily available alternative to centrifugal elutriation. Biotechniques. 2012; 53:104–108.
Article31. Tang YC, Chang HC, Roeben A, Wischnewski D, Wischnewski N, Kerner MJ, Hartl FU, Hayer-Hartl M. Structural features of the GroEL-GroES nano-cage required for rapid folding of encapsulated protein. Cell. 2006; 125:903–914.
Article32. Tu J, Lu F, Miao S, Ni X, Jiang P, Yu H, Xing L, Yu S, Ding C, Hu Q. The siderophore-interacting protein is involved in iron acquisition and virulence of Riemerella anatipestifer strain CH3. Vet Microbiol. 2014; 168:395–402.
Article33. Wang Y, Lu T, Yin X, Zhou Z, Li S, Liu M, Hu S, Bi D, Li Z. A novel RAYM_RS09735/RAYM_RS09740 two-component signaling system regulates gene expression and virulence in Riemerella anatipestifer. Front Microbiol. 2017; 8:688.
Article34. Weng S, Lin W, Chang Y, Chang C. Identification of a virulence-associated protein homolog gene and ISRa1 in a plasmid of Riemerella anatipestifer. FEMS Microbiol Lett. 1999; 179:11–19.
Article35. West AH, Stock AM. Histidine kinases and response regulator proteins in two-component signaling systems. Trends Biochem Sci. 2001; 26:369–376.
Article36. Zhou Z, Li X, Xiao Y, Wang X, Tian W, Peng X, Bi D, Sun M, Li Z. Gene expression responses to Riemerella anatipestifer infection in the liver of ducks. Avian Pathol. 2013; 42:129–136.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Two Component Signal Transduction System Required for the Virulence of Vibrio vulnificus
- The cAMP/Protein Kinase A Pathway and Virulence in Cryptococcus neoformans
- Signal Transduction
- Control of Signal Transduction Pathway
- Changes of the signal transduction system by transneuronal regulation in the olfactory bulb