Endocrinol Metab.
2018 Mar;33(1):121-132. 10.3803/EnM.2018.33.1.121.
Combined Effects of Baicalein and Docetaxel on Apoptosis in 8505c Anaplastic Thyroid Cancer Cells via Downregulation of the ERK and Akt/mTOR Pathways
- Affiliations
-
- 1Department of Internal Medicine, Ulsan University Hospital, University of Ulsan College of Medicine, Ulsan, Korea. endo10@daum.net
- KMID: 2407129
- DOI: http://doi.org/10.3803/EnM.2018.33.1.121
Abstract
- BACKGROUND
Anaplastic thyroid cancer (ATC) is one of the most lethal human malignancies. Docetaxel, a microtubule stabilizer, is a common chemotherapeutic agent used to treat various metastatic cancers. However, prolonged use results in various side effects and drug resistance. Flavonoids, such as baicalein, are accepted chemotherapeutic and dietary chemopreventive agents with many advantages, such as greater accessibility, affordability, and lower toxicity, compared with traditional chemotherapy agents. In this study, we evaluated whether baicalein enhances the effects of docetaxel on apoptosis and metastasis in 8505c ATC cells.
METHODS
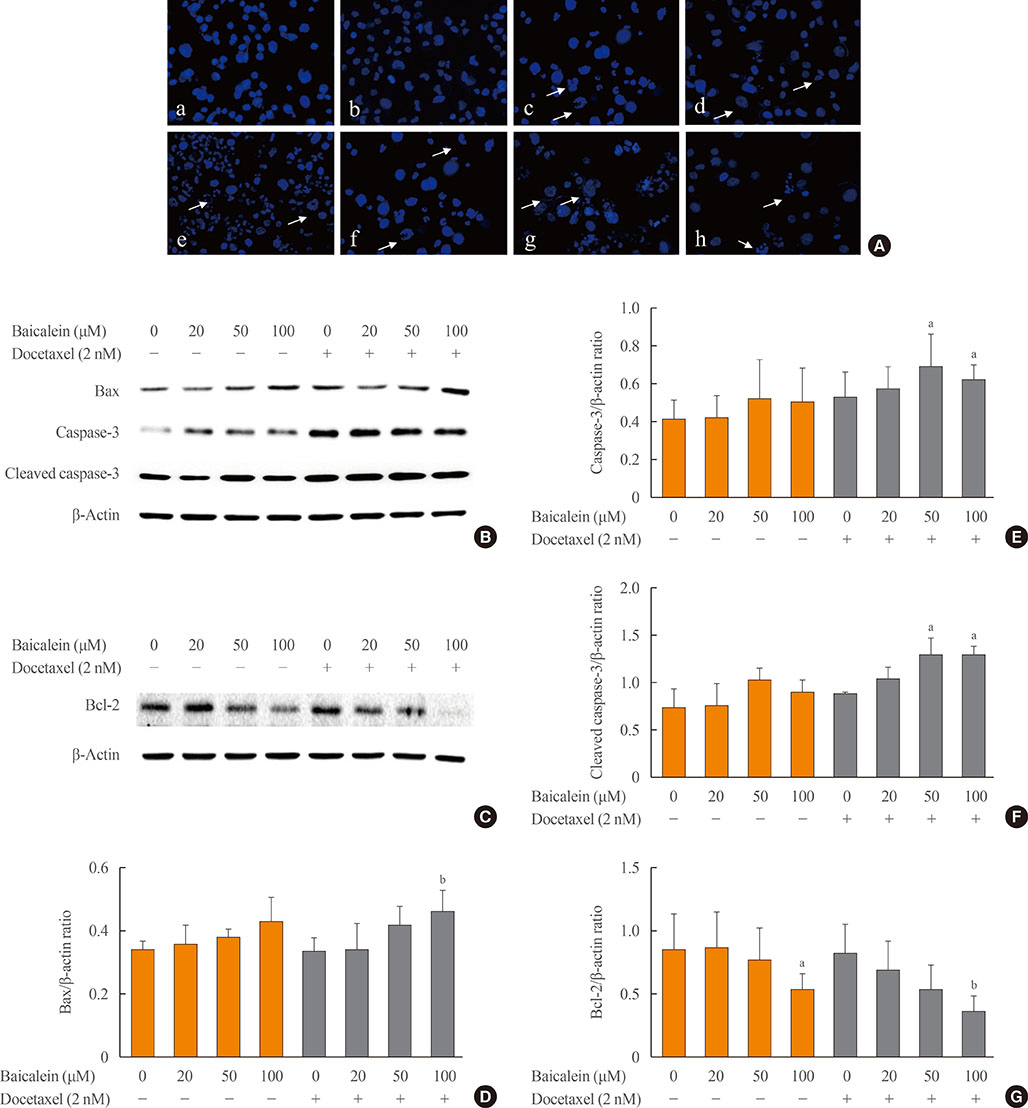
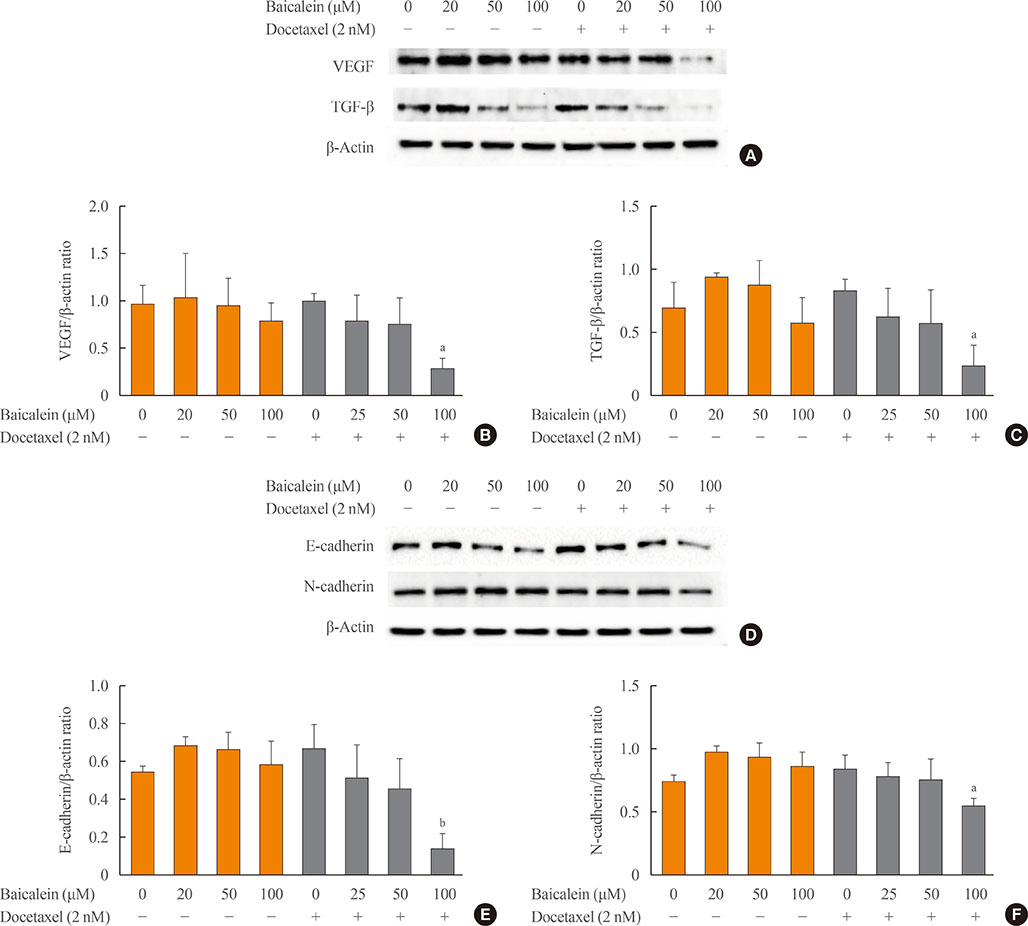
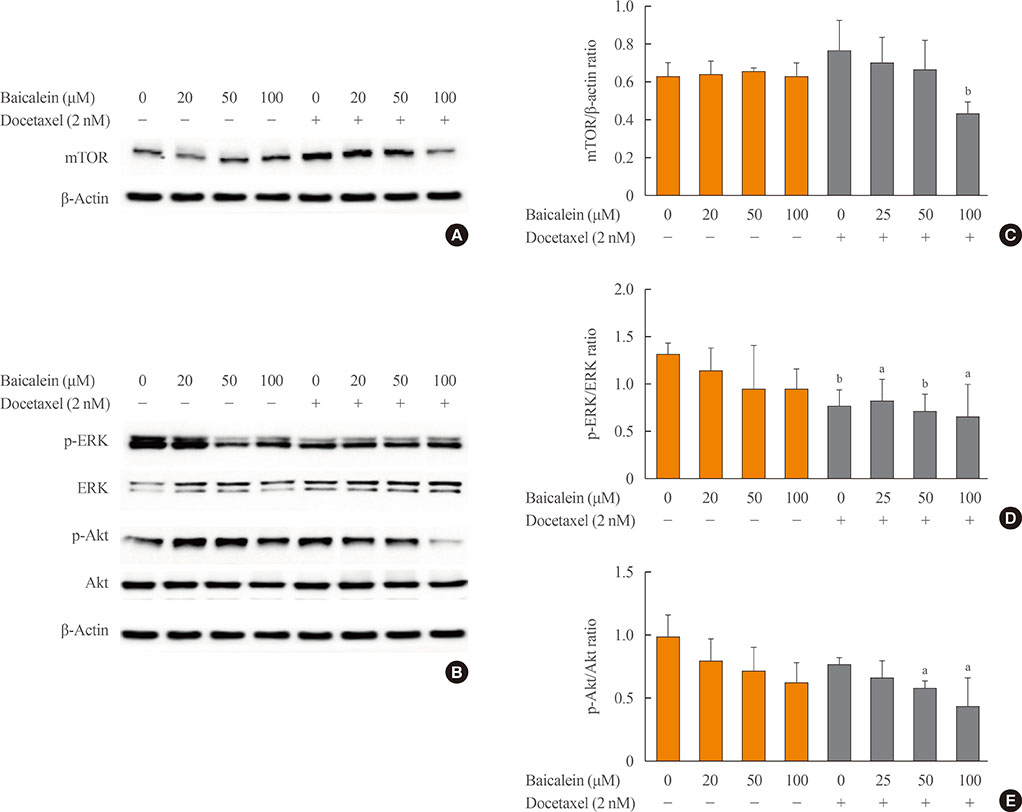
The 8505c cells were treated with baicalein or docetaxel individually and in combination. Cell viability was measured by MTT (thiazolyl blue tetrazolium bromide) assay, and apoptosis was detected by fluorescence microscopy of Hoechst-stained cells. The expression of apoptotic (Bax and caspase-3), anti-apoptotic (Bcl-2), angiogenic (vascular endothelial growth factor [VEGF], transforming growth factor β [TGF-β], E-cadherin, and N-cadherin), and signaling (extracellular signal-regulated kinase [ERK] mitogen activated protein kinase [MAPK], Akt, and mammalian target of rapamycin [mTOR]) proteins was determined by Western blot analysis.
RESULTS
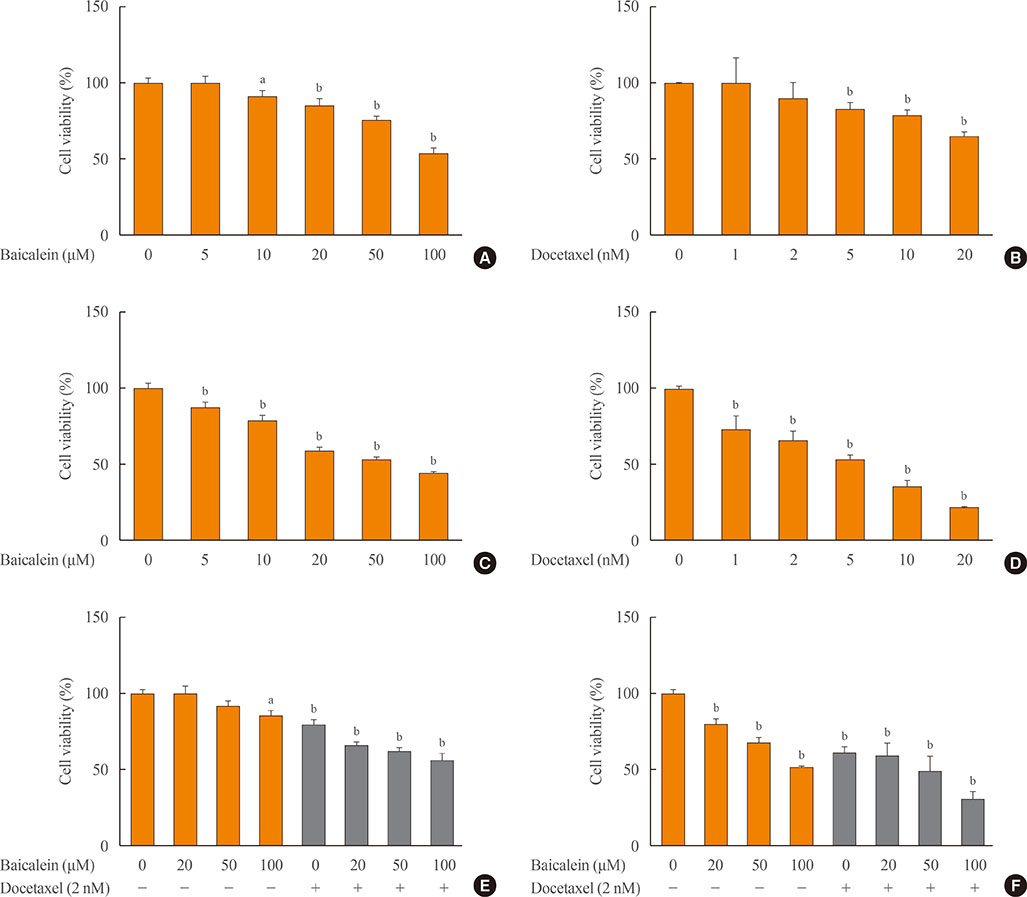
The combination of baicalein (50 or 100 µM) and docetaxel (10 nM) significantly inhibited proliferation and induced apoptosis compared with monotherapies. The combination treatment significantly inhibited the expression of Bax, caspase-3, VEGF, TGF-β1, E-cadherin, N-cadherin, and mTOR, but decreased the expression of Bcl-2 and significantly decreased the phosphorylation of ERK and Akt.
CONCLUSION
The combination of baicalein and docetaxel effectively induced apoptosis and inhibited metastasis in 8505c cells through downregulation of apoptotic and angiogenic protein expression and blocking of the ERK and Akt/mTOR pathways in 8505c cells. These results suggest that baicalein enhances the anticancer effects of docetaxel in ATC.
Keyword
MeSH Terms
-
Apoptosis*
Blotting, Western
Cadherins
Caspase 3
Cell Survival
Down-Regulation*
Drug Resistance
Drug Therapy
Endothelial Growth Factors
Flavonoids
Humans
Microscopy, Fluorescence
Microtubules
Neoplasm Metastasis
Phosphorylation
Phosphotransferases
Protein Kinases
Sirolimus
Thyroid Carcinoma, Anaplastic*
Transforming Growth Factors
Vascular Endothelial Growth Factor A
Cadherins
Caspase 3
Endothelial Growth Factors
Flavonoids
Phosphotransferases
Protein Kinases
Sirolimus
Transforming Growth Factors
Vascular Endothelial Growth Factor A
Figure
Reference
-
1. Gill KS, Tassone P, Hamilton J, Hjelm N, Luginbuhl A, Cognetti D, et al. Thyroid cancer metabolism: a review. J Thyroid Disord Ther. 2016; 5:pii200.
Article2. Keutgen XM, Sadowski SM, Kebebew E. Management of anaplastic thyroid cancer. Gland Surg. 2015; 4:44–51.3. Lim SM, Shin SJ, Chung WY, Park CS, Nam KH, Kang SW, et al. Treatment outcome of patients with anaplastic thyroid cancer: a single center experience. Yonsei Med J. 2012; 53:352–357.
Article4. Matuszczyk A, Petersenn S, Voigt W, Kegel T, Dralle H, Schmoll HJ, et al. Chemotherapy with paclitaxel and gemcitabine in progressive medullary and thyroid carcinoma of the follicular epithelium. Horm Metab Res. 2010; 42:61–64.
Article5. Bishayee A, Sethi G. Bioactive natural products in cancer prevention and therapy: progress and promise. Semin Cancer Biol. 2016; 40-41:1–3.
Article6. Spagnuolo C, Russo GL, Orhan IE, Habtemariam S, Daglia M, Sureda A, et al. Genistein and cancer: current status, challenges, and future directions. Adv Nutr. 2015; 6:408–419.
Article7. Banerjee S, Singh SK, Chowdhury I, Lillard JW Jr, Singh R. Combinatorial effect of curcumin with docetaxel modulates apoptotic and cell survival molecules in prostate cancer. Front Biosci (Elite Ed). 2017; 9:235–245.8. Mahammedi H, Planchat E, Pouget M, Durando X, Cure H, Guy L, et al. The new combination docetaxel, prednisone and curcumin in patients with castration-resistant prostate cancer: a pilot phase II study. Oncology. 2016; 90:69–78.
Article9. Makino T, Hishida A, Goda Y, Mizukami H. Comparison of the major flavonoid content of S. baicalensis, S. lateriflora, and their commercial products. J Nat Med. 2008; 62:294–299.
Article10. Takahashi H, Chen MC, Pham H, Angst E, King JC, Park J, et al. Baicalein, a component of Scutellaria baicalensis, induces apoptosis by Mcl-1 down-regulation in human pancreatic cancer cells. Biochim Biophys Acta. 2011; 1813:1465–1474.
Article11. Yan H, Xin S, Wang H, Ma J, Zhang H, Wei H. Baicalein inhibits MMP-2 expression in human ovarian cancer cells by suppressing the p38 MAPK-dependent NF-κB signaling pathway. Anticancer Drugs. 2015; 26:649–656.
Article12. Mu J, Liu T, Jiang L, Wu X, Cao Y, Li M, et al. The traditional Chinese medicine baicalein potently inhibits gastric cancer cells. J Cancer. 2016; 7:453–461.
Article13. Guo Z, Hu X, Xing Z, Xing R, Lv R, Cheng X, et al. Baicalein inhibits prostate cancer cell growth and metastasis via the caveolin-1/AKT/mTOR pathway. Mol Cell Biochem. 2015; 406:111–119.
Article14. Liu H, Dong Y, Gao Y, Du Z, Wang Y, Cheng P, et al. The fascinating effects of baicalein on cancer: a review. Int J Mol Sci. 2016; 17:E1681.
Article15. Pan Q, Xue M, Xiao SS, Wan YJ, Xu DB. A combination therapy with baicalein and taxol promotes mitochondria-mediated cell apoptosis: involving in Akt/β-catenin signaling pathway. DNA Cell Biol. 2016; 35:646–656.
Article16. Foucquier J, Guedj M. Analysis of drug combinations: current methodological landscape. Pharmacol Res Perspect. 2015; 3:e00149.
Article17. Ain KB. Management of undifferentiated thyroid cancer. Baillieres Best Pract Res Clin Endocrinol Metab. 2000; 14:615–629.
Article18. Romano B, Pagano E, Montanaro V, Fortunato AL, Milic N, Borrelli F. Novel insights into the pharmacology of flavonoids. Phytother Res. 2013; 27:1588–1596.
Article19. Yao H, Xu W, Shi X, Zhang Z. Dietary flavonoids as cancer prevention agents. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2011; 29:1–31.
Article20. Morrison JA, Pike LA, Lund G, Zhou Q, Kessler BE, Bauerle KT, et al. Characterization of thyroid cancer cell lines in murine orthotopic and intracardiac metastasis models. Horm Cancer. 2015; 6:87–99.
Article21. Shoemaker M, Cohen I, Campbell M. Reduction of MTT by aqueous herbal extracts in the absence of cells. J Ethnopharmacol. 2004; 93:381–384.
Article22. Talorete TP, Bouaziz M, Sayadi S, Isoda H. Influence of medium type and serum on MTT reduction by flavonoids in the absence of cells. Cytotechnology. 2006; 52:189–198.
Article23. Brown JM, Attardi LD. The role of apoptosis in cancer development and treatment response. Nat Rev Cancer. 2005; 5:231–237.
Article24. Zhou QM, Wang S, Zhang H, Lu YY, Wang XF, Motoo Y, et al. The combination of baicalin and baicalein enhances apoptosis via the ERK/p38 MAPK pathway in human breast cancer cells. Acta Pharmacol Sin. 2009; 30:1648–1658.
Article25. Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007; 35:495–516.
Article26. Bruncko M, Oost TK, Belli BA, Ding H, Joseph MK, Kunzer A, et al. Studies leading to potent, dual inhibitors of Bcl-2 and Bcl-xL. J Med Chem. 2007; 50:641–662.
Article27. Kim SJ, Kim HJ, Kim HR, Lee SH, Cho SD, Choi CS, et al. Antitumor actions of baicalein and wogonin in HT-29 human colorectal cancer cells. Mol Med Rep. 2012; 6:1443–1449.
Article28. Hong JM, Park CS, Nam-Goong IS, Kim YS, Lee JC, Han MW, et al. Curcumin enhances docetaxel-induced apoptosis of 8505C anaplastic thyroid carcinoma cells. Endocrinol Metab (Seoul). 2014; 29:54–61.
Article29. Mincione G, Tarantelli C, Vianale G, Di Marcantonio MC, Cotellese R, Francomano F, et al. Mutual regulation of TGF-β1, TβRII and ErbB receptors expression in human thyroid carcinomas. Exp Cell Res. 2014; 327:24–36.
Article30. Massague J, Blain SW, Lo RS. TGFbeta signaling in growth control, cancer, and heritable disorders. Cell. 2000; 103:295–309.31. Sinpitaksakul SN, Pimkhaokham A, Sanchavanakit N, Pavasant P. TGF-beta1 induced MMP-9 expression in HNSCC cell lines via Smad/MLCK pathway. Biochem Biophys Res Commun. 2008; 371:713–718.32. Sun W, Xu Y, Zhao C, Hao F, Chen D, Guan J, et al. Targeting TGF-β1 suppresses survival of and invasion by anaplastic thyroid carcinoma cells. Am J Transl Res. 2017; 9:1418–1425.33. Gulubova M, Ivanova K, Ananiev J, Gerenova J, Zdraveski A, Stoyanov H, et al. VEGF expression, microvessel density and dendritic cell decrease in thyroid cancer. Biotechnol Biotechnol Equip. 2014; 28:508–517.
Article34. Robinson CJ, Stringer SE. The splice variants of vascular endothelial growth factor (VEGF) and their receptors. J Cell Sci. 2001; 114(Pt 5):853–865.
Article35. Chen F, Zhuang M, Peng J, Wang X, Huang T, Li S, et al. Baicalein inhibits migration and invasion of gastric cancer cells through suppression of the TGF-β signaling pathway. Mol Med Rep. 2014; 10:1999–2003.
Article36. Araki K, Shimura T, Suzuki H, Tsutsumi S, Wada W, Yajima T, et al. E/N-cadherin switch mediates cancer progression via TGF-β-induced epithelial-to-mesenchymal transition in extrahepatic cholangiocarcinoma. Br J Cancer. 2011; 105:1885–1893.
Article37. Onder TT, Gupta PB, Mani SA, Yang J, Lander ES, Weinberg RA. Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res. 2008; 68:3645–3654.
Article38. Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002; 298:1911–1912.
Article39. Petrulea MS, Plantinga TS, Smit JW, Georgescu CE, Netea-Maier RT. PI3K/Akt/mTOR: a promising therapeutic target for non-medullary thyroid carcinoma. Cancer Treat Rev. 2015; 41:707–713.
Article40. Wang W, Xi M, Duan X, Wang Y, Kong F. Delivery of baicalein and paclitaxel using self-assembled nanoparticles: synergistic antitumor effect in vitro and in vivo. Int J Nanomedicine. 2015; 10:3737–3750.41. Kim SH, Shin HY, Kim YS, Kang JG, Kim CS, Ihm SH, et al. Tunicamycin induces paraptosis potentiated by inhibition of BRAFV600E in FRO anaplastic thyroid carcinoma cells. Anticancer Res. 2014; 34:4857–4868.42. Haddad RI, Lydiatt WM, Ball DW, Busaidy NL, Byrd D, Callender G, et al. Anaplastic thyroid carcinoma, version 2.2015. J Natl Compr Canc Netw. 2015; 13:1140–1150.
Article43. Hao G, Yu Y, Gu B, Xing Y, Xue M. Protective effects of berberine against doxorubicin-induced cardiotoxicity in rats by inhibiting metabolism of doxorubicin. Xenobiotica. 2015; 45:1024–1029.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Curcumin Enhances Docetaxel-Induced Apoptosis of 8505C Anaplastic Thyroid Carcinoma Cells
- Berberine Inhibited the Growth of Thyroid Cancer Cell Lines 8505C and TPC1
- Inhibition of DNMT3B and PI3K/AKT/mTOR and ERK Pathways as a Novel Mechanism of Volasertib on Hypomethylating Agent-Resistant Cells
- Myricetin Inhibits Angiogenesis by Inducing Apoptosis and Suppressing PI3K/Akt/mTOR Signaling in Endothelial Cells
- Crizotinib in Combination with Everolimus Synergistically Inhibits Proliferation of Anaplastic Lymphoma Kinase‒Positive Anaplastic Large Cell Lymphoma