Transl Clin Pharmacol.
2017 Dec;25(4):190-195. 10.12793/tcp.2017.25.4.190.
Pharmacokinetics of atorvastatin and sustained-release metformin fixed-dose combination tablets: two randomized, open-label, 2-way crossover studies in healthy male subjects under fed conditions
- Affiliations
-
- 1Department of Pharmacology and PharmacoGenomics Research Center, Inje University College of Medicine, Busan 47392, Republic of Korea.
- 2Department of Clinical Pharmacology, Inje University Busan Paik Hospital, Busan 47392, Republic of Korea. jonglyul.ghim@gmail.com
- 3CJ HealthCare Co., Ltd., Seoul 04560, Republic of Korea.
- KMID: 2407001
- DOI: http://doi.org/10.12793/tcp.2017.25.4.190
Abstract
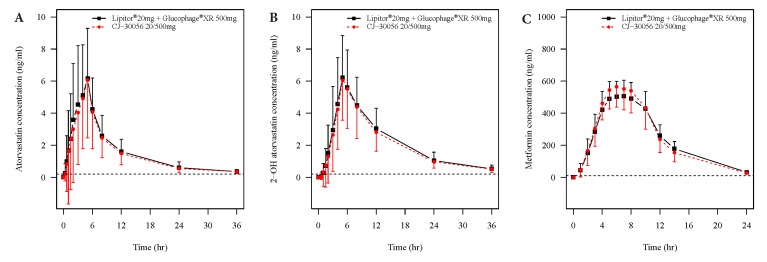
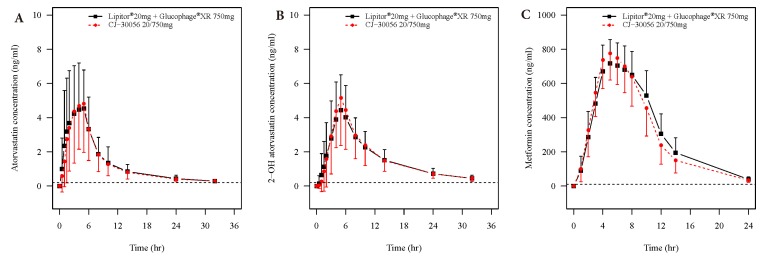
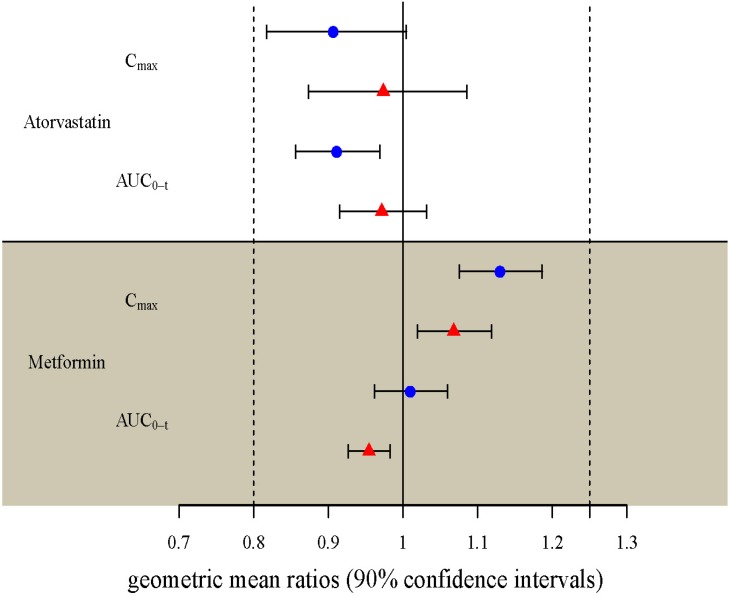
- Two separate studies were conducted to establish bioequivalence (BE) for two doses of atorvastatin/metformin sustained-release (SR) fixed dose combination (FDC) versus the same dosage of the individual component (IC) tablets in healthy male subjects under fed conditions (study 1, BE of atorvastatin/metformin SR 20/500 mg FDC; study 2, BE of atorvastatin/metformin SR 20/750 mg FDC). Each study was a randomized, open-label, single oral dose, two-way crossover design. Serial blood samples were collected pre-dose and up to 36 hours post-dose for atorvastatin and 24 hours for metformin. Plasma concentrations of atorvastatin, 2-OH atorvastatin and metformin were analyzed using a validated liquid chromatography tandem mass-spectrometry. A non-compartmental analysis was used to calculate pharmacokinetic (PK) variables and analysis of variance was performed on the lognormal-transformed PK variables. A total of 75 subjects completed the study 1 (36 subjects) and study 2 (39 subjects). The 90% confidence intervals for the adjusted geometric mean ratio of Cmax and the AUC0-t were within the predefined 0.80 to 1.25 range. The number of subjects reporting at least one adverse event following FDC treatments was comparable to that following IC treatments. The two treatments were well tolerated. Therefore, atorvastatin/metformin SR 20/500 mg and 20/750 mg FDC tablets are expected to be used as alternatives to IC tablets to decrease the pill burden and increase patient compliance.
MeSH Terms
Figure
Reference
-
1. Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, et al. , Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005; 366:1267–1278. PMID: 16214597.2. Singh IM, Shishehbor MH, Ansell BJ. High-density lipoprotein as a therapeutic target: a systematic review. JAMA. 2007; 298:786–798. PMID: 17699012.3. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Treatment of High Blood Cholesterol in, Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001; 285:2486–2497. PMID: 11368702.4. Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, et al. , Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004; 364:685–696. PMID: 15325833.5. Collins R, Armitage J, Parish S, Sleigh P, Peto R, Heart Protection. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet. 2003; 361:2005–2016. PMID: 12814710.6. Johnson JA, Majumdar SR, Simpson SH, Toth EL. Decreased mortality associated with the use of metformin compared with sulfonylurea monotherapy in type 2 diabetes. Diabetes Care. 2002; 25:2244–2248. PMID: 12453968.
Article7. UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998; 352:854–865. PMID: 9742977.8. Zangiabadi N, Shafiee K, Alavi KH, Assadi AR, Damavandi M. Atorvastatin treatment improves diabetic polyneuropathy electrophysiological changes in non-insulin dependent diabetic patients: a double blind, randomized clinical trial. Minerva Endocrinol. 2012; 37:195–200. PMID: 22691892.9. Athyros VG, Karagiannis A, Katsiki N, Mikhailidis DP. Relation of improvement in glomerular filtration rate with atorvastatin to reductions in heart failure morbidity. Am J Cardiol. 2012; 110:763. DOI: 10.1016/j.amjcard.2012.06.007.
Article10. Lehman DM, Lorenzo C, Hernandez J, Wang CP. Statin use as a moderator of metformin effect on risk for prostate cancer among type 2 diabetic patients. Diabetes Care. 2012; 35:1002–1007. PMID: 22456867.
Article11. Scheen AJ. Drug interactions of clinical importance with antihyperglycaemic agents: an update. Drug Saf. 2005; 28:601–631.12. Oh JH, Eun Lee J, Jeong Kim Y, Oh TO, Han S, Jeon EK, et al. , Designing of the fixed-dose gastroretentive bilayer tablet for sustained release of metformin and immediate release of atorvastatin. Drug Dev Ind Pharm. 2016; 42:340–349. DOI: 10.3109/03639045.2015.1096279. PMID: 26467296.13. Liu YM, Pu HH, Liu GY, Jia JY, Weng LP, Xu RJ, et al. , Pharmacokinetics and bioequivalence evaluation of two different atorvastatin calcium 10-mg tablets: A single-dose, randomized-sequence, open-label, two-period crossover study in healthy fasted Chinese adult males. Clin Ther. 2010; 32:1396–1407. DOI: 10.1016/j.clinthera.2010.07.004. PMID: 20678686.14. Wang Y, Tang Y, Gu J, Fawcett JP, Bai X. Rapid and sensitive liquid chromatography-tandem mass spectrometric method for the quantitation of metformin in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2004; 808:215–219.
Article15. He BX, Shi L, Qiu J, Zeng XH, Tao L, Li R, et al. Quantitative determination of atorvastatin and ortho-hydroxy atorvastatin in human plasma by liquid chromatography tandem mass spectrometry and pharmacokinetic evaluation. Methods Find Exp Clin Pharmacol. 2010; 32:481–487. PMID: 21069099.
Article16. Gong L, Goswami S, Giacomini KM, Altman RB, Klein TE. Metformin pathways: pharmacokinetics and pharmacodynamics. Pharmacogenet Genomics. 2012; 22:820–827. DOI: 10.1097/FPC.0b013e3283559b22. PMID: 22722338.17. Lennernas H. Clinical pharmacokinetics of atorvastatin. Clin Pharmacokinet. 2003; 42:1141–1160. PMID: 14531725.18. Lea AP, McTavish D. Atorvastatin. A review of its pharmacology and therapeutic potential in the management of hyperlipidaemias. Drugs. 1997; 53:828–847. PMID: 9129869.19. García-Pérez LE, Alvarez M, Dilla T, Gil-Guillén V, Orozco-Beltrán D. Adherence to therapies in patients with type 2 diabetes. Diabetes Ther. 2013; 4:175–194. DOI: 10.1007/s13300-013-0034-y. PMID: 23990497.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pharmacokinetics and pharmacodynamics of a fixed-dose combination of gemigliptin/metformin sustained release 25/500 mg compared to the loose combination in healthy male subjects
- Pharmacokinetic Equivalence of the High Dose Strength Fixed-Dose Combination Tablet of Gemigliptin/Metformin Sustained Release (SR) and Individual Component Gemigliptin and Metformin XR Tablets in Healthy Subjects
- Fed and fasted bioequivalence assessment of two formulations of extended-release fixed-dose combination dapagliflozin/metformin (10/1,000 mg) tablets in healthy subjects
- A randomized, open-label, single-dose, two-way crossover study to assess the pharmacokinetics between two tablets of fixed-dose combination formulation with raloxifene and cholecalciferol and concomitant administration of each agents in healthy male volunteers
- Pharmacokinetics of fixed-dose combination of rosuvastatin 20 mg and ezetimibe 10 mg compared to concurrent administration of individual tablets in healthy Korean subjects