Transl Clin Pharmacol.
2017 Dec;25(4):173-178. 10.12793/tcp.2017.25.4.173.
Development of a simple and sensitive HPLC-MS/MS method for determination of diazepam in human plasma and its application to a bioequivalence study
- Affiliations
-
- 1Department of Life and Nanopharmaceutical Sciences, Graduate School, Kyung Hee University, 26 Kyungheedae-ro, Dongdaemun-gu, Seoul 02447, Korea. ktlee@khu.ac.kr
- 2Kyung Hee Drug Analysis Center, Kyung Hee University, 26 Kyungheedae-ro, Dongdaemun-gu, Seoul 02447, Korea.
- 3Department of Pharmacy, College of Pharmacy, Kyung Hee University, 26 Kyungheedae-ro, Dongdaemun-gu, Seoul 02447, Korea.
- KMID: 2406998
- DOI: http://doi.org/10.12793/tcp.2017.25.4.173
Abstract
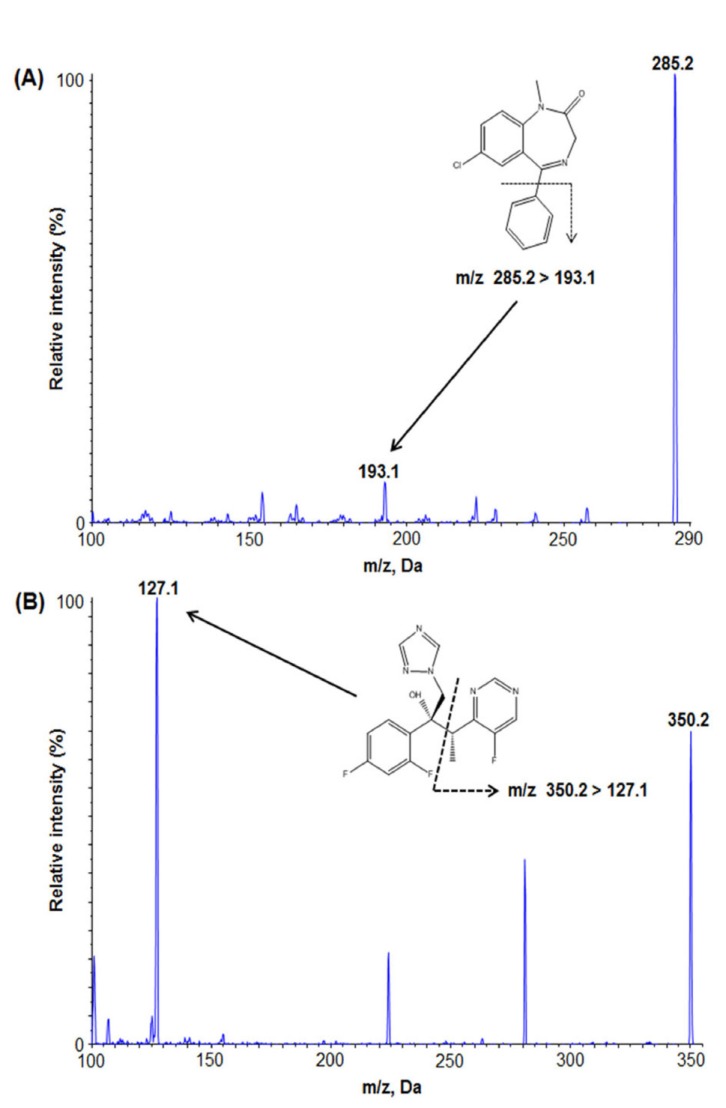
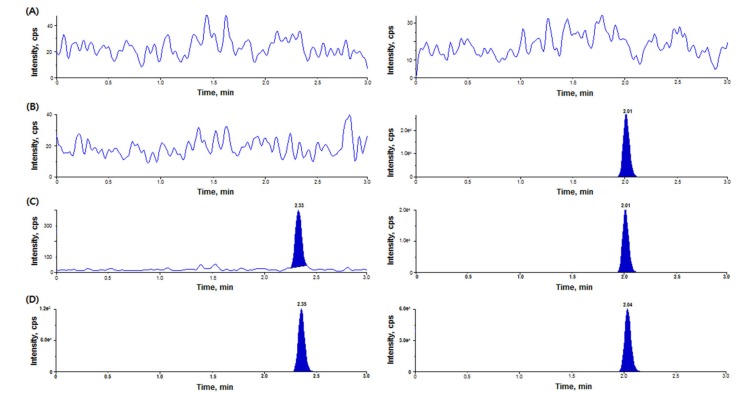
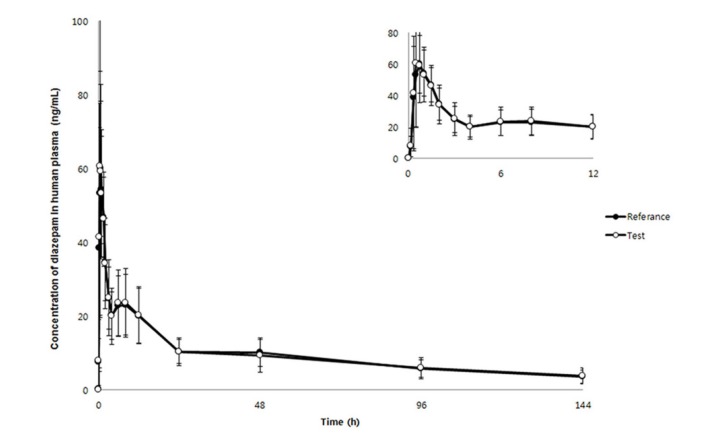
- We developed a simple, sensitive, and effective ultra-performance liquid chromatography/tandem mass spectrometry (HPLC-MS/MS) method with an electrospray ionization (ESI) interface in multiple reaction monitoring (MRM) and positive ion modes to determine diazepam concentrations in human plasma using voriconazole as an internal standard (IS). Diazepam and IS were detected at transition 285.2→193.1 and 350.2→127.1, respectively. After liquid-liquid extraction (LLE) using 1.2 ml of ethyl acetate:n-hexane (80:20, v/v), diazepam and IS were eluted on a Phenomenex Cadenza CD-C18 column (150 × 3.0 mm, 3 µm) with an isocratic mobile phase (10 mM ammonium acetate in water:methanol [5:95, v/v]) at a flow rate of 0.4 mL/min. The peak retention time was 2.32 min for diazepam and 2.01 min for IS, respectively. The lower limit of quantitation (LLOQ) was 0.5 ng/mL (S/N > 10) using 50 µL of plasma, and no interferences were observed in chromatograms. Our analytical method was fully validated and successfully applied to a bioequivalence study of two formulations of diazepam in healthy Korean volunteers.
Keyword
MeSH Terms
Figure
Reference
-
1. Klotz U, Avant GR, Hoyumpa A, Schenker S, Wilkinson GR. The effects of age and liver disease on the disposition and elimination of diazepam in adult man. J Clin Invest. 1975; 55:347–359. PMID: 1127104.
Article2. APO-diazepam-productinformation-Australia. Accessed 10 September 2017. http://www.medicines.org.au/files/txpdiaze.pdf.3. Rouini MR, Ardakani YH, Moghaddam KA, Solatani F. An improved HPLC method for rapid quantitation of diazepam and its major metabolites in human plasma. Talanta. 2008; 75:671–676. DOI: 10.1016/j.talanta.2007.11.060. PMID: 18585130.
Article4. Mercolini L, Mandrioli R, Iannello C, Matrisciano F, Nicoletti F, Raggi MA. Simultaneous analysis of diazepam and its metabolites in rat plasma and brain tissue by HPLC-UV and SPE. Talanta. 2009; 80:279–285. DOI: 10.1016/j.talanta.2009.06.074. PMID: 19782227.
Article5. Agarwal SK, Kriel RL, Brundage RC, Ivaturi VD, Cloyd JC. A pilot study assessing the bioavailability and pharmacokinetics of diazepam after intranasal and intravenous administration in healthy volunteers. Epilepsy Res. 2013; 105:362–362. DOI: 10.1016/j.eplepsyres.2013.02.018. PMID: 23561287.
Article6. Lee XP, Shouji Y, Kumazawa T, Hasegawa C, Fujishiro M, Sato J, et al. Rapid and highly sensitive analysis of benzodiazepines and tandospirone in human plasma by automated on-line column-switching UFLC-MS/MS. Leg Med (Tokyo). 2017; 24:36–55. DOI: 10.1016/j.legalmed.2016.11.005. PMID: 28081789.
Article7. Wang R, Wang X, Liang C, Ni C, Xiong L, Rao Y, et al. Direct determination of diazepam and its glucuronide metabolites in human whole blood by μElution solid-phase extraction and liquid chromatography–tandem mass spectrometry. Forensic Sci Int. 2013; 233:304–311. DOI: 10.1016/j.forsciint.2013.10.004. PMID: 24314534.
Article8. Jiang F, Rao Y, Wang R, Johansen SS, Ni C, Liang C, et al. Sensitive, automatic method for the determination of diazepam and its five metabolites in human oral fluid by online solid-phase extraction and liquid chromatography with tandem mass spectrometry. J Sep Sci. 2016; 39:1873–1883. DOI: 10.1002/jssc.201600107. PMID: 27005561.
Article9. De Boeck M, Missotten S, Dehaen W, Tytgat J, Cuypers E. Development and validation of a fast ionic liquid-based dispersive liquid–liquid microextraction procedure combined with LC–MS/MS analysis for the quantification of benzodiazepines and benzodiazepine-like hypnotics in whole blood. Forensic Sci Int. 2017; 274:44–54. DOI: 10.1016/j.forsciint.2016.12.026. PMID: 28094153.
Article10. Ivaturi V, Kriel R, Brundage R, Loewen G, Mansbach H, Cloyd J. Bioavailability of intranasal vs. rectal diazepam. Epilepsy Res. 2013; 103:254–261. DOI: 10.1016/j.eplepsyres.2012.07.018. PMID: 22981338.
Article11. Abbara C, Bardot I, Cailleux A, Lallement G, Le Bouil A, Turcant A, et al. High-performance liquid chromatography coupled with electrospray tandem mass spectrometry (LC/MS/MS) method for the simultaneous determination of diazepam, atropine and pralidoxime in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2008; 874:42–50. DOI: 10.1016/j.jchromb.2008.08.027.
Article12. Cloyd JC, Lalonde RL, Beniak TE, Novack GD. A Single-Blind, crossover comparison of the pharmacokinetics and cognitive effects of a new diazepam rectal gel with intravenous diazepam. Epilepsia. 1998; 39:520–526. PMID: 9596205.
Article13. Abbara C, Rousseau J, Turcant A, Lallement G, Comets E, Bardot I, et al. Bioavailability of diazepam after intramuscular injection of its water-soluble prodrug alone or with atropine–pralidoxime in healthy volunteers. Br J Pharmacol. 2009; 157:1390–1397. DOI: 10.1111/j.1476-5381.2009.00330.x. PMID: 19681868.
Article14. Ivaturi VD, Riss JR, Kriel RL, Cloyd JC. Pharmacokinetics and tolerability of intranasal diazepam and midazolam in healthy adult volunteers. Acta Neurol Scand. 2009; 120:353–357. DOI: 10.1111/j.1600-0404.2009.01170.x. PMID: 19456308.
Article15. Lamson MJ, Sitki-Green D, Wannarka GL, Mesa M, Andrews P, Pellock J. Pharmacokinetics of diazepam administered intramuscularly by autoinjector versus rectal gel in healthy subjects: a phase I, randomized, open-label, single-dose, crossover, single-centre study. Clin Drug Investig. 2011; 31:585–597. DOI: 10.2165/11590250-000000000-00000.16. Bio-analytical Method Validation. Korea Food and Drug Administration;2013. Accessed 20 September 2017. https://eirb.ajoumc.or.kr/board/file/Notice/Notice_1055.pdf.17. Guidance for industry: Bioanalytical method validation. Accessed 30 September 2017. https://www.fda.gov/downloads/drugs/guidances/ucm368107.pdf.18. General Assembly of the World Medical Association. World medical association declaration of helsinki: Ethical principles for medical research involving human subjects. J Am Coll Dent. 2014; 81:14–18. PMID: 25951678.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Development and validation of analytical method for the determination of radotinib in human plasma using liquid chromatography-tandem mass spectrometry
- Ultrafast liquid chromatographytandem mass spectrometry determination of donepezil in human plasma: application to a bioequivalence study
- Determination of sumatriptan in human plasma using liquid chromatography-mass spectrometry for pharmacokinetic study in healthy Korean volunteers
- Development of a LC-MS/MS for Quantification of Venlafaxine in Human Plasma and Application to Bioequivalence Study in healthy Korean Subjects
- Development and validation of LC-MS/MS for bioanalysis of hydroxychloroquine in human whole blood