J Periodontal Implant Sci.
2018 Feb;48(1):12-21. 10.5051/jpis.2018.48.1.12.
An in vitro model of Fusobacterium nucleatum and Porphyromonas gingivalis in single- and dual-species biofilms
- Affiliations
-
- 1Department of Dental Materials and Prosthodontics, São Paulo State University - UNESP School of Dentistry at Araraquara, Araraquara, Sao Paulo, Brazil. pavarina@foar.unesp.br
- KMID: 2405406
- DOI: http://doi.org/10.5051/jpis.2018.48.1.12
Abstract
- PURPOSE
The goal of this study was to develop and validate a standardized in vitro pathogenic biofilm attached onto saliva-coated surfaces.
METHODS
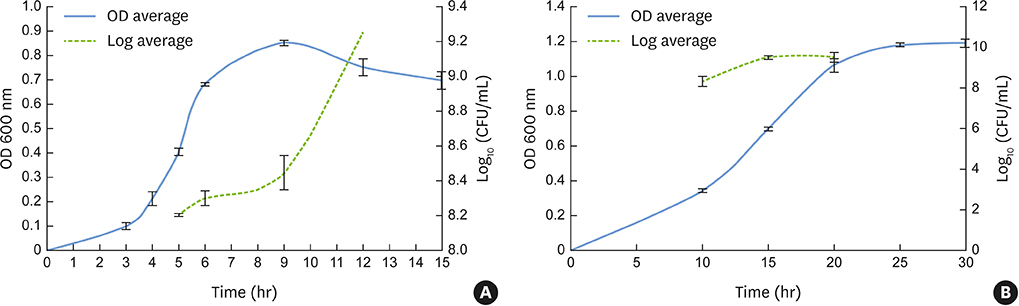
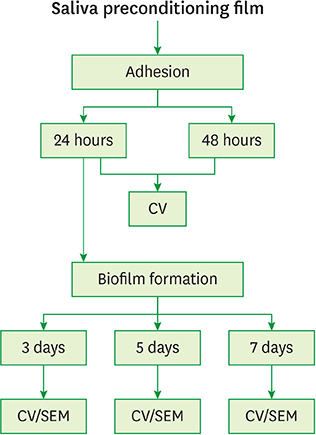
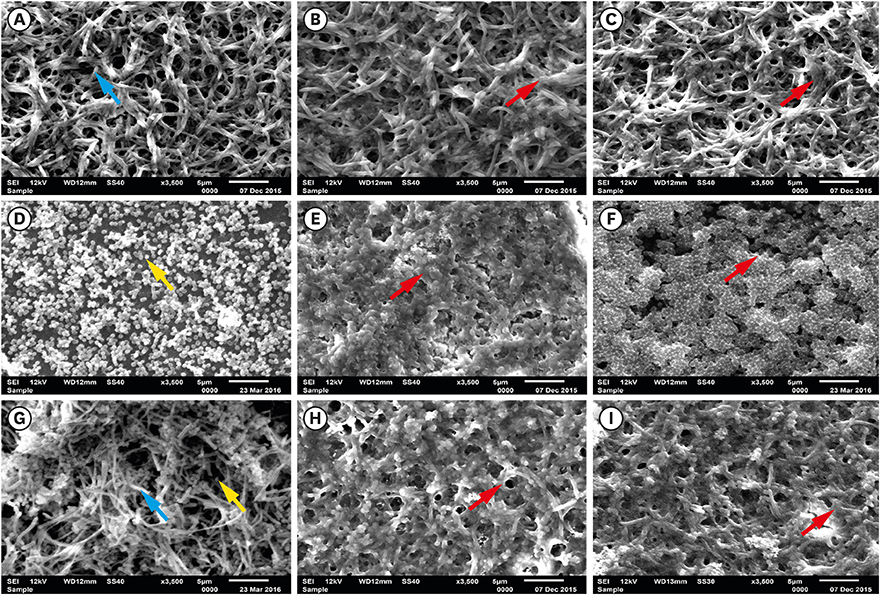
Fusobacterium nucleatum (F. nucleatum) and Porphyromonas gingivalis (P. gingivalis) strains were grown under anaerobic conditions as single species and in dual-species cultures. Initially, the bacterial biomass was evaluated at 24 and 48 hours to determine the optimal timing for the adhesion phase onto saliva-coated polystyrene surfaces. Thereafter, biofilm development was assessed over time by crystal violet staining and scanning electron microscopy.
RESULTS
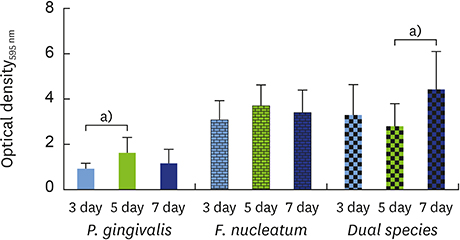
The data showed no significant difference in the overall biomass after 48 hours for P. gingivalis in single- and dual-species conditions. After adhesion, P. gingivalis in single- and dual-species biofilms accumulated a substantially higher biomass after 7 days of incubation than after 3 days, but no significant difference was found between 5 and 7 days. Although the biomass of the F. nucleatum biofilm was higher at 3 days, no difference was found at 3, 5, or 7 days of incubation.
CONCLUSIONS
Polystyrene substrates from well plates work as a standard surface and provide reproducible results for in vitro biofilm models. Our biofilm model could serve as a reference point for studies investigating biofilms on different surfaces.
MeSH Terms
Figure
Reference
-
1. Malcolm J, Millington O, Millhouse E, Campbell L, Adrados Planell A, Butcher JP, et al. Mast cells contribute to Porphyromonas gingivalis-induced bone loss. J Dent Res. 2016; 95:704–710.
Article2. Feng X, Zhu L, Xu L, Meng H, Zhang L, Ren X, et al. Distribution of 8 periodontal microorganisms in family members of Chinese patients with aggressive periodontitis. Arch Oral Biol. 2015; 60:400–407.
Article3. Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJ. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res. 2012; 91:914–920.
Article4. Marcenes W, Kassebaum NJ, Bernabe E, Flaxman A, Naghavi M, Lopez A, et al. Global burden of oral conditions in 1990–2010: a systematic analysis. J Dent Res. 2013; 92:592–597.5. Zitzmann NU, Berglundh T. Definition and prevalence of peri-implant diseases. J Clin Periodontol. 2008; 35:286–291.
Article6. Socransky SS, Haffajee AD. Periodontal microbial ecology. Periodontol 2000. 2005; 38:135–187.
Article7. How KY, Song KP, Chan KG. Porphyromonas gingivalis: an overview of periodontopathic pathogen below the gum line. Front Microbiol. 2016; 7:53.
Article8. van Winkelhoff AJ, Loos BG, van der Reijden WA, van der Velden U. Porphyromonas gingivalis, Bacteroides forsythus and other putative periodontal pathogens in subjects with and without periodontal destruction. J Clin Periodontol. 2002; 29:1023–1028.
Article9. Periasamy S, Kolenbrander PE. Mutualistic biofilm communities develop with Porphyromonas gingivalis and initial, early, and late colonizers of enamel. J Bacteriol. 2009; 191:6804–6811.
Article10. Tribble GD, Kerr JE, Wang BY. Genetic diversity in the oral pathogen Porphyromonas gingivalis: molecular mechanisms and biological consequences. Future Microbiol. 2013; 8:607–620.
Article11. Kirschbaum M, Schultze-Mosgau S, Pfister W, Eick S. Mixture of periodontopathogenic bacteria influences interaction with KB cells. Anaerobe. 2010; 16:461–468.
Article12. Saito A, Kokubu E, Inagaki S, Imamura K, Kita D, Lamont RJ, et al. Porphyromonas gingivalis entry into gingival epithelial cells modulated by Fusobacterium nucleatum is dependent on lipid rafts. Microb Pathog. 2012; 53:234–242.
Article13. Feuille F, Ebersole JL, Kesavalu L, Stepfen MJ, Holt SC. Mixed infection with Porphyromonas gingivalis and Fusobacterium nucleatum in a murine lesion model: potential synergistic effects on virulence. Infect Immun. 1996; 64:2094–2100.
Article14. Metzger Z, Lin YY, Dimeo F, Ambrose WW, Trope M, Arnold RR. Synergistic pathogenicity of Porphyromonas gingivalis and Fusobacterium nucleatum in the mouse subcutaneous chamber model. J Endod. 2009; 35:86–94.
Article15. Diaz PI, Zilm PS, Rogers AH. Fusobacterium nucleatum supports the growth of Porphyromonas gingivalis in oxygenated and carbon-dioxide-depleted environments. Microbiology. 2002; 148:467–472.
Article16. Ahn SH, Song JE, Kim S, Cho SH, Lim YK, Kook JK, et al. NOX1/2 activation in human gingival fibroblasts by Fusobacterium nucleatum facilitates attachment of Porphyromonas gingivalis. Arch Microbiol. 2016; 198:573–583.
Article17. Periasamy S, Chalmers NI, Du-Thumm L, Kolenbrander PE. Fusobacterium nucleatum ATCC 10953 requires Actinomyces naeslundii ATCC 43146 for growth on saliva in a three-species community that includes Streptococcus oralis 34. Appl Environ Microbiol. 2009; 75:3250–3257.
Article18. Ebersole JL, Feuille F, Kesavalu L, Holt SC. Host modulation of tissue destruction caused by periodontopathogens: effects on a mixed microbial infection composed of Porphyromonas gingivalis and Fusobacterium nucleatum . Microb Pathog. 1997; 23:23–32.
Article19. Kumada Y, Benson DR, Hillemann D, Hosted TJ, Rochefort DA, Thompson CJ, et al. Evolution of the glutamine synthetase gene, one of the oldest existing and functioning genes. Proc Natl Acad Sci USA. 1993; 90:3009–3013.
Article20. Amoroso PF, Adams RJ, Waters MG, Williams DW. Titanium surface modification and its effect on the adherence of Porphyromonas gingivalis: an in vitro study. Clin Oral Implants Res. 2006; 17:633–637.
Article21. de Avila ED, Avila-Campos MJ, Vergani CE, Spolidorio DM, Mollo Fde A Jr. Structural and quantitative analysis of a mature anaerobic biofilm on different implant abutment surfaces. J Prosthet Dent. 2016; 115:428–436.
Article22. de Avila ED, Lima BP, Sekiya T, Torii Y, Ogawa T, Shi W, et al. Effect of UV-photofunctionalization on oral bacterial attachment and biofilm formation to titanium implant material. Biomaterials. 2015; 67:84–92.
Article23. Montelongo-Jauregui D, Srinivasan A, Ramasubramanian AK, Lopez-Ribot JL. An in vitro model for oral mixed biofilms of Candida albicans and Streptococcus gordonii in synthetic saliva. Front Microbiol. 2016; 7:686.
Article24. Barros J, Grenho L, Fontenente S, Manuel CM, Nunes OC, Melo LF, et al. Staphylococcus aureus and Escherichia coli dual-species biofilms on nanohydroxyapatite loaded with CHX or ZnO nanoparticles. J Biomed Mater Res A. 2017; 105:491–497.
Article25. Liu Y, Busscher HJ, Zhao B, Li Y, Zhang Z, van der Mei HC, et al. Surface-adaptive, antimicrobially loaded, micellar nanocarriers with enhanced penetration and killing efficiency in staphylococcal biofilms. ACS Nano. 2016; 10:4779–4789.
Article26. Malaikozhundan B, Vaseeharan B, Vijayakumar S, Pandiselvi K, Kalanjiam MA, Murugan K, et al. Biological therapeutics of Pongamia pinnata coated zinc oxide nanoparticles against clinically important pathogenic bacteria, fungi and MCF-7 breast cancer cells. Microb Pathog. 2017; 104:268–277.
Article27. Badihi Hauslich L, Sela MN, Steinberg D, Rosen G, Kohavi D. The adhesion of oral bacteria to modified titanium surfaces: role of plasma proteins and electrostatic forces. Clin Oral Implants Res. 2013; 24:Suppl A100. 49–56.
Article28. Kohavi D, Klinger A, Steinberg D, Mann E, Sela NM. Alpha-amylase and salivary albumin adsorption onto titanium, enamel and dentin: an in vivo study. Biomaterials. 1997; 18:903–906.
Article29. Moura JS, da Silva WJ, Pereira T, Del Bel Cury AA, Rodrigues Garcia RC. Influence of acrylic resin polymerization methods and saliva on the adherence of four Candida species. J Prosthet Dent. 2006; 96:205–211.
Article30. Pereira-Cenci T, Cury AA, Cenci MS, Rodrigues-Garcia RC. In vitro Candida colonization on acrylic resins and denture liners: influence of surface free energy, roughness, saliva, and adhering bacteria. Int J Prosthodont. 2007; 20:308–310.31. Sánchez MC, Llama-Palacios A, Blanc V, León R, Herrera D, Sanz M. Structure, viability and bacterial kinetics of an in vitro biofilm model using six bacteria from the subgingival microbiota. J Periodontal Res. 2011; 46:252–260.
Article32. Thein ZM, Samaranayake YH, Samaranayake LP. Characteristics of dual species Candida biofilms on denture acrylic surfaces. Arch Oral Biol. 2007; 52:1200–1208.
Article33. Ciandrini E, Campana R, Federici S, Manti A, Battistelli M, Falcieri E, et al. In vitro activity of Carvacrol against titanium-adherent oral biofilms and planktonic cultures. Clin Oral Investig. 2014; 18:2001–2013.
Article34. Navarro Llorens JM, Tormo A, Martinez-Garcia E. Stationary phase in gram-negative bacteria. FEMS Microbiol Rev. 2010; 34:476–495.
Article35. Rolfe MD, Rice CJ, Lucchini S, Pin C, Thompson A, Cameron AD, et al. Lag phase is a distinct growth phase that prepares bacteria for exponential growth and involves transient metal accumulation. J Bacteriol. 2012; 194:686–701.
Article36. Kim KS, Anthony BF. Importance of bacterial growth phase in determining minimal bactericidal concentrations of penicillin and methicillin. Antimicrob Agents Chemother. 1981; 19:1075–1077.
Article37. de Avila ED, de Molon R, Vergani CE, Mollo FA Jr, Salih V. The relationship between biofilm and physical-chemical properties of implant abutment materials for successful dental implants. Materials (Basel). 2014; 7:3651–3662.
Article38. de Avila ED, de Molon R, Spolidorio DM, Mollo FA Jr. Implications of surface and bulk properties of abutment implants and their degradation in the health of periodontal tissue. Materials (Basel). 2013; 6:5951–5966.
Article39. de Avila ED, Vergani CE, Mollo FA Junior, Junior MJ, Shi W, Lux R. Effect of titanium and zirconia dental implant abutments on a cultivable polymicrobial saliva community. J Prosthet Dent. 2017; 118:481–487.
Article40. de Avila ED, de Molon RS, Lima BP, Lux R, Shi W, Junior MJ, et al. Impact of physical chemical characteristics of abutment implant surfaces on bacteria adhesion. J Oral Implantol. 2016; 42:153–158.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Fusobacterium nucleatum modulates serum binding to Porphyromonas gingivalis biofilm
- A periodontitis-associated multispecies model of an oral biofilm
- Prior Immunization with Fusobacterium Nucleatum Interferes with Opsonophagocytosis Function of Sera against Porphyromonas Gingivalis
- Perturbation of host responses by Porphyromonas gingivalis biofilm
- Effects of Microbial Communication on The Growth of Periodontopathogens