Clin Endosc.
2018 Jan;51(1):103-108. 10.5946/ce.2017.093.
Full-Thickness Resection Device for Complex Colorectal Lesions in High-Risk Patients as a Last-Resort Endoscopic Treatment: Initial Clinical Experience and Review of the Current Literature
- Affiliations
-
- 1Department of Gastroenterology and Gastrointestinal Oncology, University Medical Center Göttingen, Göttingen, Germany. edris.wedi@med.uni-goettingen.de
- 2Department of Gastroenterology, Careggi University Hospital, Florence, Italy.
- 3Division of Gastroenterology, Indiana University School of Medicine, Indianapolis, IN, USA.
- 4Department of Pathology, Nouvel Hôpital Civil, Strasbourg University Hospitals, Strasbourg, France.
- 5Department of Internal Medicine, Vivantes Klinikum im Friedrichshain, University Teaching Hospital of Humboldt University Berlin (Charité), Berlin, Germany.
- KMID: 2403641
- DOI: http://doi.org/10.5946/ce.2017.093
Abstract
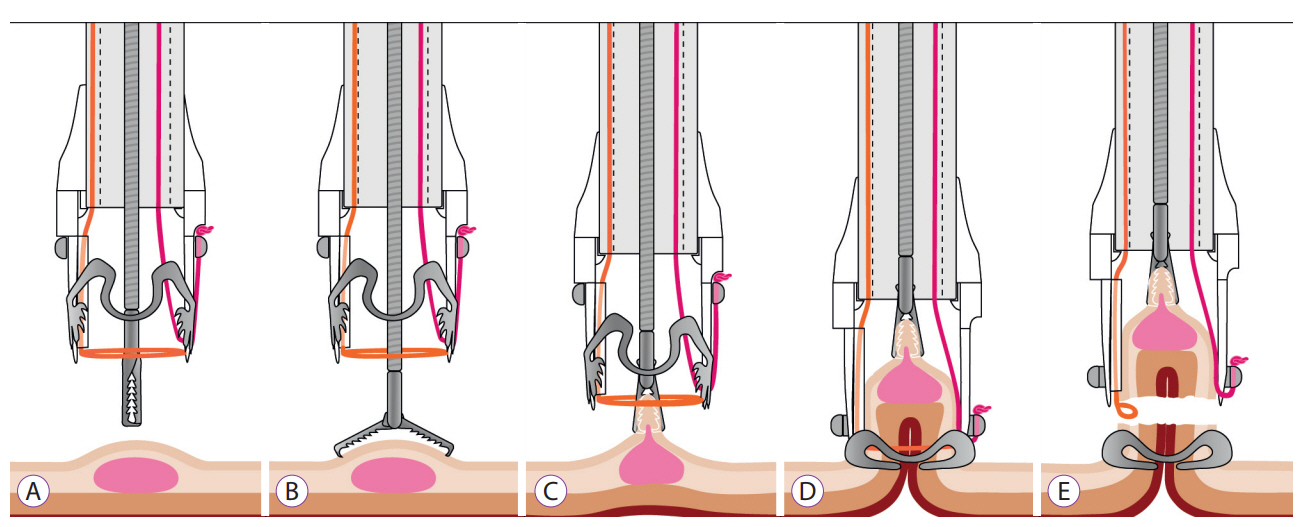
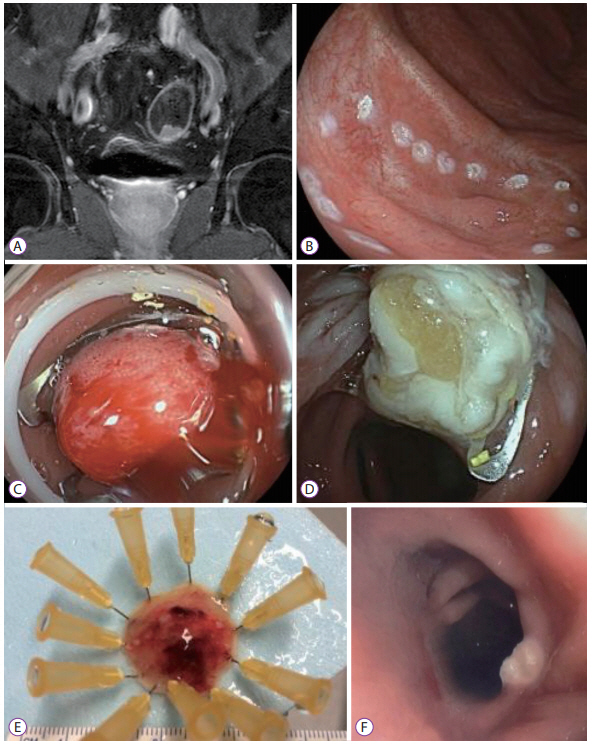
- The full-thickness resection device (FTRD) is a novel endoscopic device approved for the resection of colorectal lesions. This case-series describes the device and its use in high-risk patients with colorectal lesions and provides an overview of the potential indications in recently published data. Between December 2014 and September 2015, 3 patients underwent endoscopic full thickness resection using the FTRD for colorectal lesions: 1 case for a T1 adenocarcinoma in the region of a surgical anastomosis after recto-sigmoidectomy, 1 case for a non-lifting colonic adenoma with low-grade dysplasia in an 89-year old patient and 1 for a recurrent adenoma with high-grade dysplasia in a young patient with ulcerative rectocolitis who was under immunosuppression after renal transplantation. Both technical and clinical success rates were achieved in all cases. The size of removed lesions ranged from 9 to 30 mm. Overall, the most frequent indication in the literature has been for lifting or non-lifting adenoma, submucosal tumors, neuroendocrin tumors, incomplete endoscopic resection (R1) or T1 carcinoma. Colorectal FTRD is a feasible technique for the treatment of colorectal lesions and represents a minimally invasive alternative for either surgical or conventional endoscopic resection strategies.
MeSH Terms
Figure
Cited by 1 articles
-
Technical Feasibility of a Guidetube for Various Endoscopic Procedures in Human Gastrointestinal Simulators
Dong Seok Lee, Byeong Gwan Kim, Kook Lae Lee, Yong Jin Jung, Ji Won Kim
Clin Endosc. 2019;52(3):247-251. doi: 10.5946/ce.2018.147.
Reference
-
1. Hochberger J, Köhler P, Kruse E, et al. [Endoscopic submucosal dissection]. Internist (Berl). 2013; 54:287–301.2. von Renteln D, Rösch T, Kratt T, Denzer UW, El-Masry M, Schachschal G. Endoscopic full-thickness resection of submucosal gastric tumors. Dig Dis Sci. 2012; 57:1298–1303.
Article3. Schurr MO, Baur F, Ho CN, Anhoeck G, Kratt T, Gottwald T. Endoluminal full-thickness resection of GI lesions: a new device and technique. Minim Invasive Ther Allied Technol. 2011; 20:189–192.
Article4. Hotta K, Oyama T, Shinohara T, et al. Learning curve for endoscopic submucosal dissection of large colorectal tumors. Dig Endosc. 2010; 22:302–306.
Article5. Fähndrich M, Sandmann M. Endoscopic full-thickness resection for gastrointestinal lesions using the over-the-scope clip system: a case series. Endoscopy. 2015; 47:76–79.
Article6. Mönkemüller K, Peter S, Toshniwal J, et al. Multipurpose use of the ‘bear claw’ (over-the-scope-clip system) to treat endoluminal gastrointestinal disorders. Dig Endosc. 2014; 26:350–357.
Article7. Sarker S, Gutierrez JP, Council L, Brazelton JD, Kyanam Kabir Baig KR, Mönkemüller K. Over-the-scope clip-assisted method for resection of full-thickness submucosal lesions of the gastrointestinal tract. Endoscopy. 2014; 46:758–761.
Article8. Richter-Schrag HJ, Walker C, Thimme R, Fischer A. [Full thickness resection device (FTRD). Experience and outcome for benign neoplasms of the rectum and colon]. Chirurg. 2016; 87:316–325.9. Schmidt A, Meier B, Cahyadi O, Caca K. Duodenal endoscopic full-thickness resection (with video). Gastrointest Endosc. 2015; 82:728–733.
Article10. Schmidt A, Bauerfeind P, Gubler C, Damm M, Bauder M, Caca K. Endoscopic full-thickness resection in the colorectum with a novel overthe- scope device: first experience. Endoscopy. 2015; 47:719–725.11. Schmidt A, Damm M, Caca K. Endoscopic full-thickness resection using a novel over-the-scope device. Gastroenterology. 2014; 147:740–742. e2.
Article12. Valli PV, Kaufmann M, Vrugt B, Bauerfeind P. Endoscopic resection of a diverticulum-arisen colonic adenoma using a full-thickness resection device. Gastroenterology. 2014; 147:969–971.
Article13. Klare P, Burlefinger R, Neu B, et al. Over-the-scope clip-assisted endoscopic full-thickness resection after incomplete resection of a rectal euroendocrine tumor. Endoscopy. 2015; 47(Suppl 1):UCTN:E47–E48.14. Eriksen TF, Lassen CB, Gögenur I. Treatment with corticosteroids and the risk of anastomotic leakage following lower gastrointestinal surgery: a literature survey. Colorectal Dis. 2014; 16:O154–O160.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Endoscopic Full-Thickness Resection for Gastric Subepithelial Lesions Arising from the Muscularis Propria
- Challenges in Implementing Endoscopic Resection for T2 Colorectal Cancer
- Endoscopic Full-thickness Resection for Gastric Tumor
- Risk Stratification of T1 Colorectal Cancer Metastasis to Lymph Nodes: Current Status and Perspective
- Closing the Gaps: Endoscopic Suturing for Large Submucosal and Full-Thickness Defects