Cancer Res Treat.
2018 Jan;50(1):293-301. 10.4143/crt.2016.543.
Second Primary Cancer Risk among Kidney Cancer Patients in Korea: A Population-Based Cohort Study
- Affiliations
-
- 1Center for Prostate Cancer, National Cancer Center, Goyang, Korea.
- 2Department of Urology, Institute of Wonkwang Medical Science, Wonkwang University Sanbon Hospital, Wonkwang University School of Medicine, Gunpo, Korea.
- 3Cancer Registration and Statistics Branch, National Cancer Center, Goyang, Korea. astra67@ncc.re.kr
- KMID: 2403498
- DOI: http://doi.org/10.4143/crt.2016.543
Abstract
- PURPOSE
Secondary primary cancers (SPCs) commonly arise in patients with renal cell carcinoma (RCC). We designed the present study to estimate the SPC incidence in Korean patients with RCC.
MATERIALS AND METHODS
The study cohort was population-based and consisted of 40,347 individuals from the Korean Central Cancer Registry who were diagnosed with primary renal cancer between 1993 and 2013. Standardized incidence ratios (SIRs) for SPCs were estimated for different ages at diagnosis, latencies, diagnostic periods, and treatments.
RESULTS
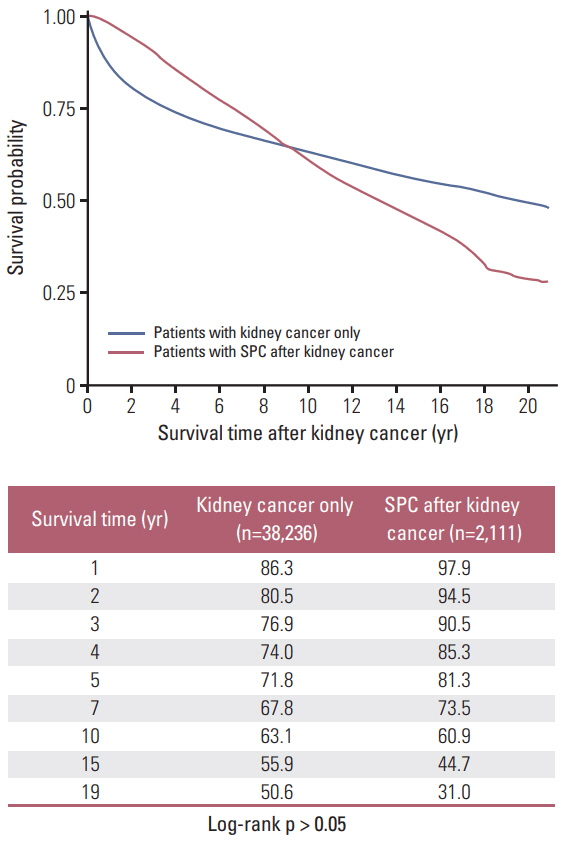
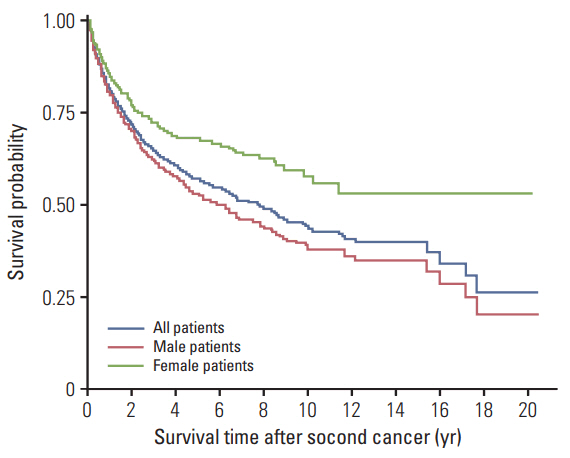
For patients with primary RCC, the risk of developing a SPC was higher than the risk of developing cancer in the general population (SIR, 1.13; 95% confidence interval, 1.08 to 1.18). Most cancer types showed higher incidences in patients with RCC than in the general population. However, the relative incidence of gastric cancer as an SPC varied by age. Gastric cancer incidence was elevated in young patients (< 30 years) with RCC, but reduced in older (≥ 30) patients with RCC. Patients with advanced RCC died prematurely, regardless of SPC development. In contrast, those with early-stage RCC survived for longer periods, although SPC development affected their post-RCC survival. After SPC development, women had better survival than men.
CONCLUSION
In Korean patients with primary RCC, the incidence of SPC was 13% higher than the incidence of cancer in the general population. These findings may play important roles in the conduct of follow-up evaluations and education for patients with RCC.
MeSH Terms
Figure
Reference
-
References
1. Travis LB. The epidemiology of second primary cancers. Cancer Epidemiol Biomarkers Prev. 2006; 15:2020–6.
Article2. Wood ME, Vogel V, Ng A, Foxhall L, Goodwin P, Travis LB. Second malignant neoplasms: assessment and strategies for risk reduction. J Clin Oncol. 2012; 30:3734–45.
Article3. Ljungberg B, Hanbury DC, Kuczyk MA, Merseburger AS, Mulders PF, Patard JJ, et al. Renal cell carcinoma guideline. Eur Urol. 2007; 51:1502–10.
Article4. Oh CM, Won YJ, Jung KW, Kong HJ, Cho H, Lee JK, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2013. Cancer Res Treat. 2016; 48:436–50.
Article5. Chen T, Fallah M, Sundquist K, Liu H, Hemminki K. Risk of subsequent cancers in renal cell carcinoma survivors with a family history. Eur J Cancer. 2014; 50:2108–18.
Article6. Liu H, Hemminki K, Sundquist J. Renal cell carcinoma as first and second primary cancer: etiological clues from the Swedish Family-Cancer Database. J Urol. 2011; 185:2045–9.
Article7. Hemminki K, Boffetta P. Multiple primary cancers as clues to environmental and heritable causes of cancer and mechanisms of carcinogenesis. IARC Sci Publ. 2004; (157):289–97.8. Travis LB, Rabkin CS, Brown LM, Allan JM, Alter BP, Ambrosone CB, et al. Cancer survivorship: genetic susceptibility and second primary cancers: research strategies and recommendations. J Natl Cancer Inst. 2006; 98:15–25.9. Rixe O, Rini B. Renal cell carcinoma: ten years of significant advances. Target Oncol. 2010; 5:73–4.
Article10. Rini BI, Campbell SC, Rathmell WK. Renal cell carcinoma. Curr Opin Oncol. 2006; 18:289–96.
Article11. Shin HR, Won YJ, Jung KW, Kong HJ, Yim SH, Lee JK, et al. Nationwide cancer incidence in Korea, 1999~2001; first result using the national cancer incidence database. Cancer Res Treat. 2005; 37:325–31.
Article12. Curado MP, Edwards B, Shin HR, Storm H, Ferlay J, Heanue M, et al. Cancer incidence in five continents. Vol. 9. Lyon: IARC Press;2007.13. Fritz A, Percy C, Jack A, Shanmugaratnam K, Sobin L, Parkin DM, et al. International classification of diseases for oncology. 3rd ed. Geneva: World Health Organization;2000.14. Cho YY, Lim J, Oh CM, Ryu J, Jung KW, Chung JH, et al. Elevated risks of subsequent primary malignancies in patients with thyroid cancer: a nationwide, population-based study in Korea. Cancer. 2015; 121:259–68.
Article15. International Agency for Research on Cancer; World Health Organization; International Association of Cancer Registries; European Network of Cancer Registries. International rules for multiple primary cancers (ICD-O third edition). Lyon: International Agency for Research on Cancer;2004.16. Curtis RE, Freedman DM, Ron E, Ries LA, Hacker DG, Edwards BK, et al. New malignancies among cancer survivors: SEER cancer registries, 1973-2000. NIH Publ. No. 05-5302. Bethesda, MD: National Cancer Institute;2006.17. Esteve J, Benhamou E, Raymond L. Statistical methods in cancer research. Vol. IV. Descriptive epidemiology. IARC scentific publication. No. 128. Lyon: International Agency for Research on Cancer;1994.18. Tsugane S. Salt, salted food intake, and risk of gastric cancer: epidemiologic evidence. Cancer Sci. 2005; 96:1–6.
Article19. Tsugane S, Sasazuki S, Kobayashi M, Sasaki S. Salt and salted food intake and subsequent risk of gastric cancer among middle-aged Japanese men and women. Br J Cancer. 2004; 90:128–34.
Article20. Chakraborty S, Tarantolo SR, Batra SK, Hauke RJ. Incidence and prognostic significance of second primary cancers in renal cell carcinoma. Am J Clin Oncol. 2013; 36:132–42.
Article21. Beisland C, Talleraas O, Bakke A, Norstein J. Multiple primary malignancies in patients with renal cell carcinoma: a national population-based cohort study. BJU Int. 2006; 97:698–702.
Article22. Rabbani F, Reuter VE, Katz J, Russo P. Second primary malignancies associated with renal cell carcinoma: influence of histologic type. Urology. 2000; 56:399–403.
Article23. Thompson RH, Leibovich BC, Cheville JC, Webster WS, Lohse CM, Kwon ED, et al. Second primary malignancies associated with renal cell carcinoma histological subtypes. J Urol. 2006; 176:900–3.
Article24. Sun M, Lughezzani G, Perrotte P, Karakiewicz PI. Treatment of metastatic renal cell carcinoma. Nat Rev Urol. 2010; 7:327–38.
Article25. Mataraza JM, Gotwals P. Recent advances in immuno-oncology and its application to urological cancers. BJU Int. 2016; 118:506–14.
Article26. Davey RJ, van der Westhuizen A, Bowden NA. Metastatic melanoma treatment: combining old and new therapies. Crit Rev Oncol Hematol. 2016; 98:242–53.
Article27. Hu-Lieskovan S, Mok S, Homet Moreno B, Tsoi J, Robert L, Goedert L, et al. Improved antitumor activity of immunotherapy with BRAF and MEK inhibitors in BRAF(V600E) melanoma. Sci Transl Med. 2015; 7:279ra41.
Article28. Massari F, Santoni M, Ciccarese C, Santini D, Alfieri S, Martignoni G, et al. PD-1 blockade therapy in renal cell carcinoma: current studies and future promises. Cancer Treat Rev. 2015; 41:114–21.
Article29. Mahoney KM, Freeman GJ, McDermott DF. The next immune-checkpoint inhibitors: PD-1/PD-L1 blockade in melanoma. Clin Ther. 2015; 37:764–82.
Article30. Carosella ED, Ploussard G, LeMaoult J, Desgrandchamps F. A systematic review of immunotherapy in urologic cancer: evolving roles for targeting of CTLA-4, PD-1/PD-L1, and HLA-G. Eur Urol. 2015; 68:267–79.
Article31. Rubino C, de Vathaire F, Dottorini ME, Hall P, Schvartz C, Couette JE, et al. Second primary malignancies in thyroid cancer patients. Br J Cancer. 2003; 89:1638–44.
Article32. Ronckers CM, McCarron P, Ron E. Thyroid cancer and multiple primary tumors in the SEER cancer registries. Int J Cancer. 2005; 117:281–8.
Article33. Berthe E, Henry-Amar M, Michels JJ, Rame JP, Berthet P, Babin E, et al. Risk of second primary cancer following differentiated thyroid cancer. Eur J Nucl Med Mol Imaging. 2004; 31:685–91.
Article34. Edmonds CJ, Smith T. The long-term hazards of the treatment of thyroid cancer with radioiodine. Br J Radiol. 1986; 59:45–51.
Article35. Akslen LA, Glattre E. Second malignancies in thyroid cancer patients: a population-based survey of 3658 cases from Norway. Eur J Cancer. 1992; 28:491–5.
Article36. Malchoff CD, Sarfarazi M, Tendler B, Forouhar F, Whalen G, Joshi V, et al. Papillary thyroid carcinoma associated with papillary renal neoplasia: genetic linkage analysis of a distinct heritable tumor syndrome. J Clin Endocrinol Metab. 2000; 85:1758–64.
Article37. Cohen HT, McGovern FJ. Renal-cell carcinoma. N Engl J Med. 2005; 353:2477–90.
Article38. Fioroni I, Dell'Aquila E, Pantano F, Intagliata S, Caricato M, Vincenzi B, et al. Role of c-mesenchymal-epithelial transition pathway in gastric cancer. Expert Opin Pharmacother. 2015; 16:1195–207.
Article39. Peng Z, Zhu Y, Wang Q, Gao J, Li Y, Li Y, et al. Prognostic significance of MET amplification and expression in gastric cancer: a systematic review with meta-analysis. PLoS One. 2014; 9:e84502.
Article40. Miettinen M, Lasota J. Succinate dehydrogenase deficient gastrointestinal stromal tumors (GISTs): a review. Int J Biochem Cell Biol. 2014; 53:514–9.41. Niemeijer ND, Papathomas TG, Korpershoek E, de Krijger RR, Oudijk L, Morreau H, et al. Succinate dehydrogenase (SDH)-deficient pancreatic neuroendocrine tumor expands the SDH-related tumor spectrum. J Clin Endocrinol Metab. 2015; 100:E1386–93.
Article42. Doyle LA, Nelson D, Heinrich MC, Corless CL, Hornick JL. Loss of succinate dehydrogenase subunit B (SDHB) expression is limited to a distinctive subset of gastric wild-type gastrointestinal stromal tumours: a comprehensive genotype-phenotype correlation study. Histopathology. 2012; 61:801–9.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Metformin Use and Gastric Cancer Risk
- Green Tea Consumption and Stomach Cancer Risk in Women: A Meta-analysis of Population-Based Cohort Studies
- Risk of Second Primary Cancer in People with Non-melanoma Skin Cancer: A Nationwide Cohort Study
- History of Diabetes Mellitus and Risk of Breast Cancer in Asian Women: A Meta-Epidemiological Analysis of Population-Based Cohort Studies
- Risk of Second Primary Malignancy in Breast Cancer Survivors: A Nested Population-Based Case-Control Study