Cancer Res Treat.
2018 Jan;50(1):239-254. 10.4143/crt.2016.580.
FOXO1 Suppression is a Determinant of Acquired Lapatinib-Resistance in HER2-Positive Gastric Cancer Cells Through MET Upregulation
- Affiliations
-
- 1Tumour Biology, Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea. dslanat@snu.ac.kr
- 2Department of Forensic Medicine, National Forensic Service Busan Institute, Yangsan, Korea.
- 3Department of Pathology, Seoul National University College of Medicine, Seoul, Korea.
- 4Department of Pathology, Eulji University Hospital, Eulji University School of Medicine, Daejeon, Korea.
- 5Department of Pathology, Jeju National University Hospital, Jeju, Korea.
- 6Department of Molecular Medicine, Inha University College of Medicine, Incheon, Korea.
- 7Ischemic/Hypoxic Disease Institute Medical Research Center, Seoul National University College of Medicine, Seoul, Korea.
- 8Department of Anatomy, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2403493
- DOI: http://doi.org/10.4143/crt.2016.580
Abstract
- PURPOSE
Lapatinib is a candidate drug for treatment of trastuzumab-resistant, human epidermal growth factor receptor 2 (HER2)-positive gastric cancer (GC). Unfortunately, lapatinib resistance renders this drug ineffective. The present study investigated the implication of forkhead box O1 (FOXO1) signaling in the acquired lapatinib resistance in HER2-positive GC cells.
MATERIALS AND METHODS
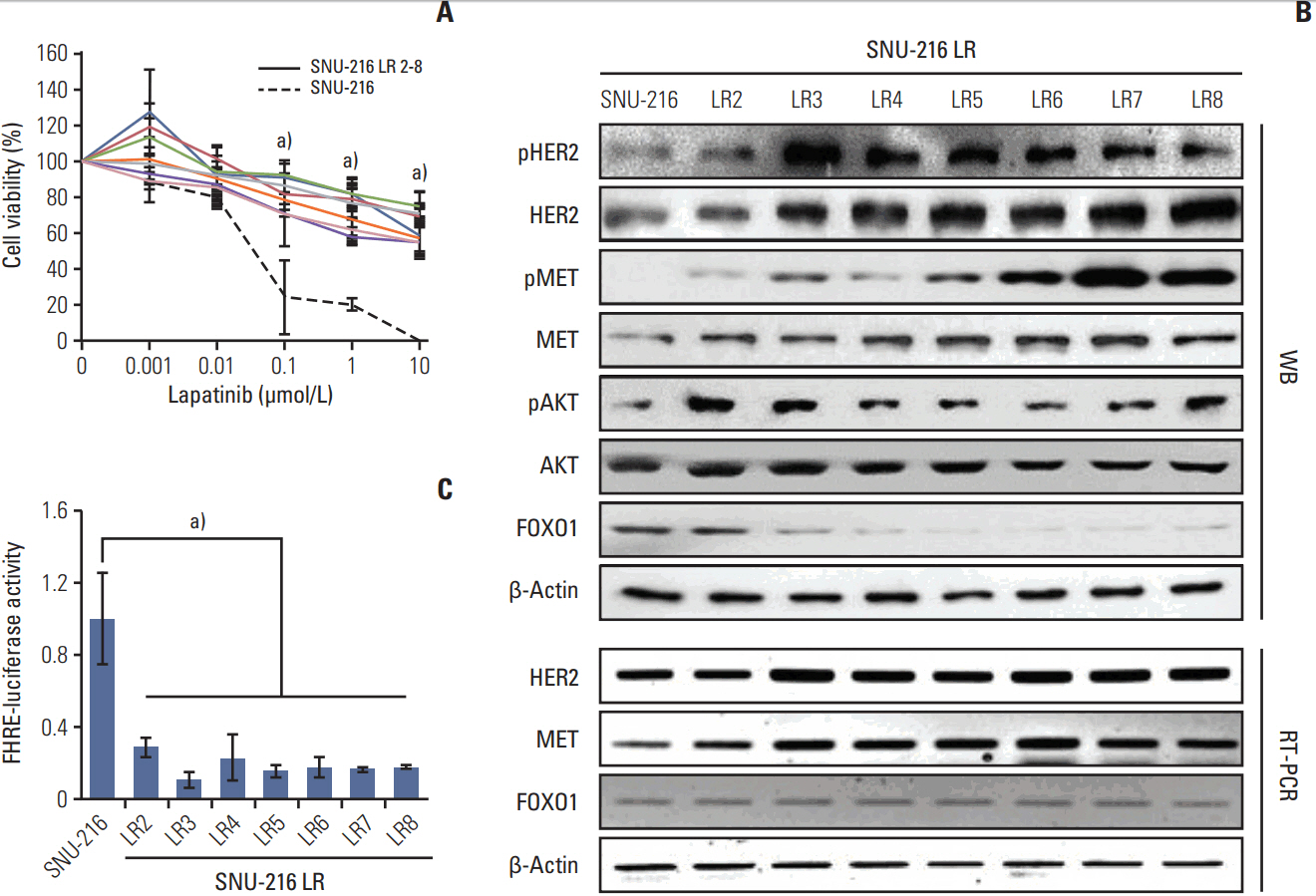
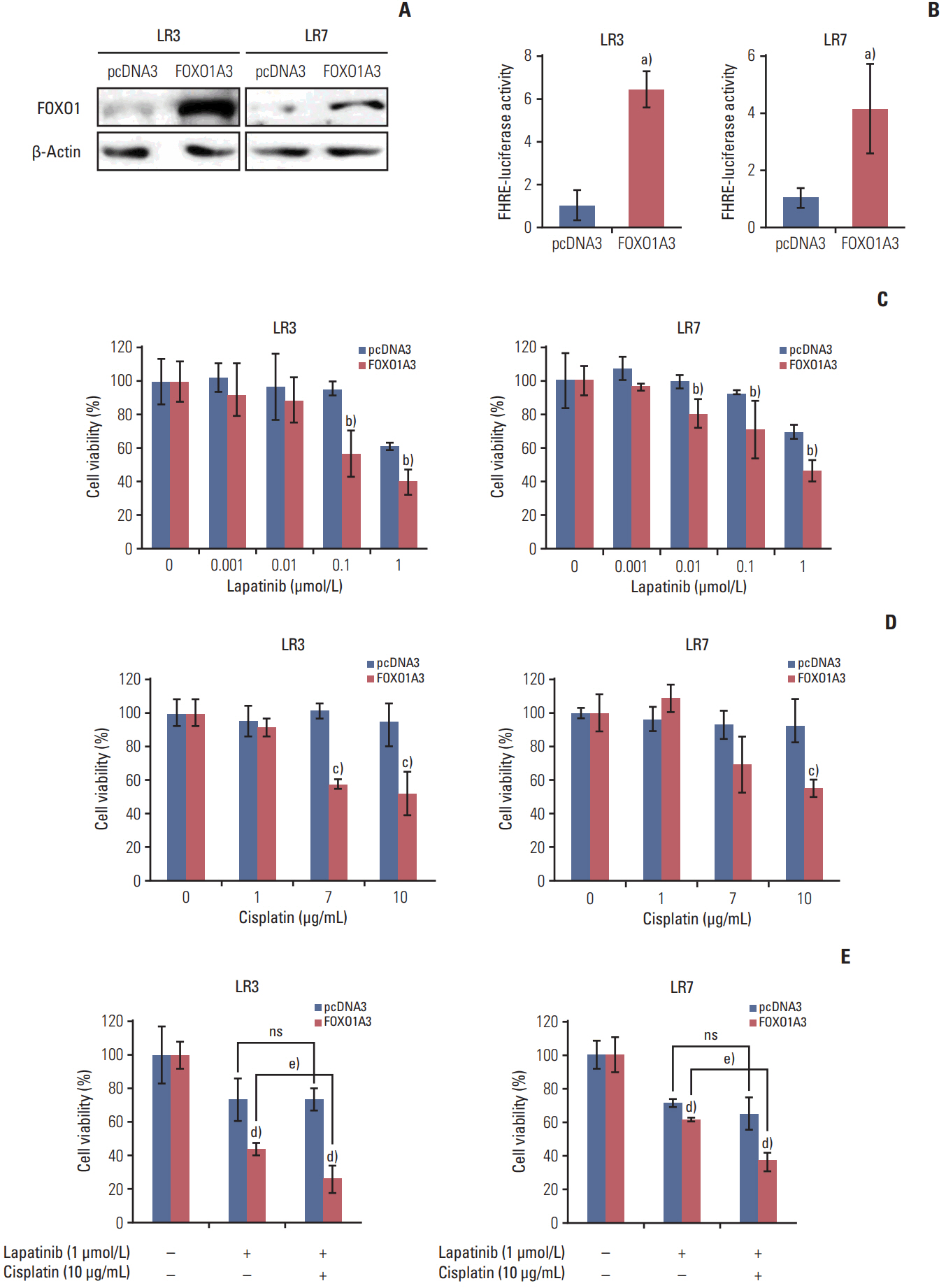
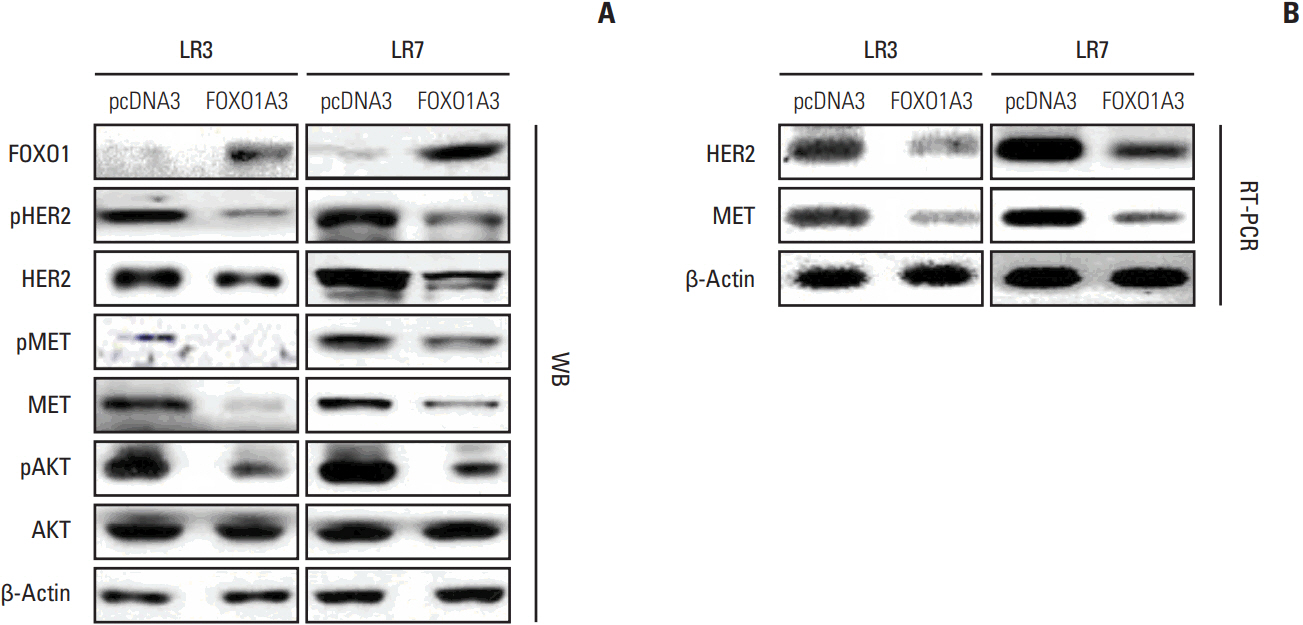
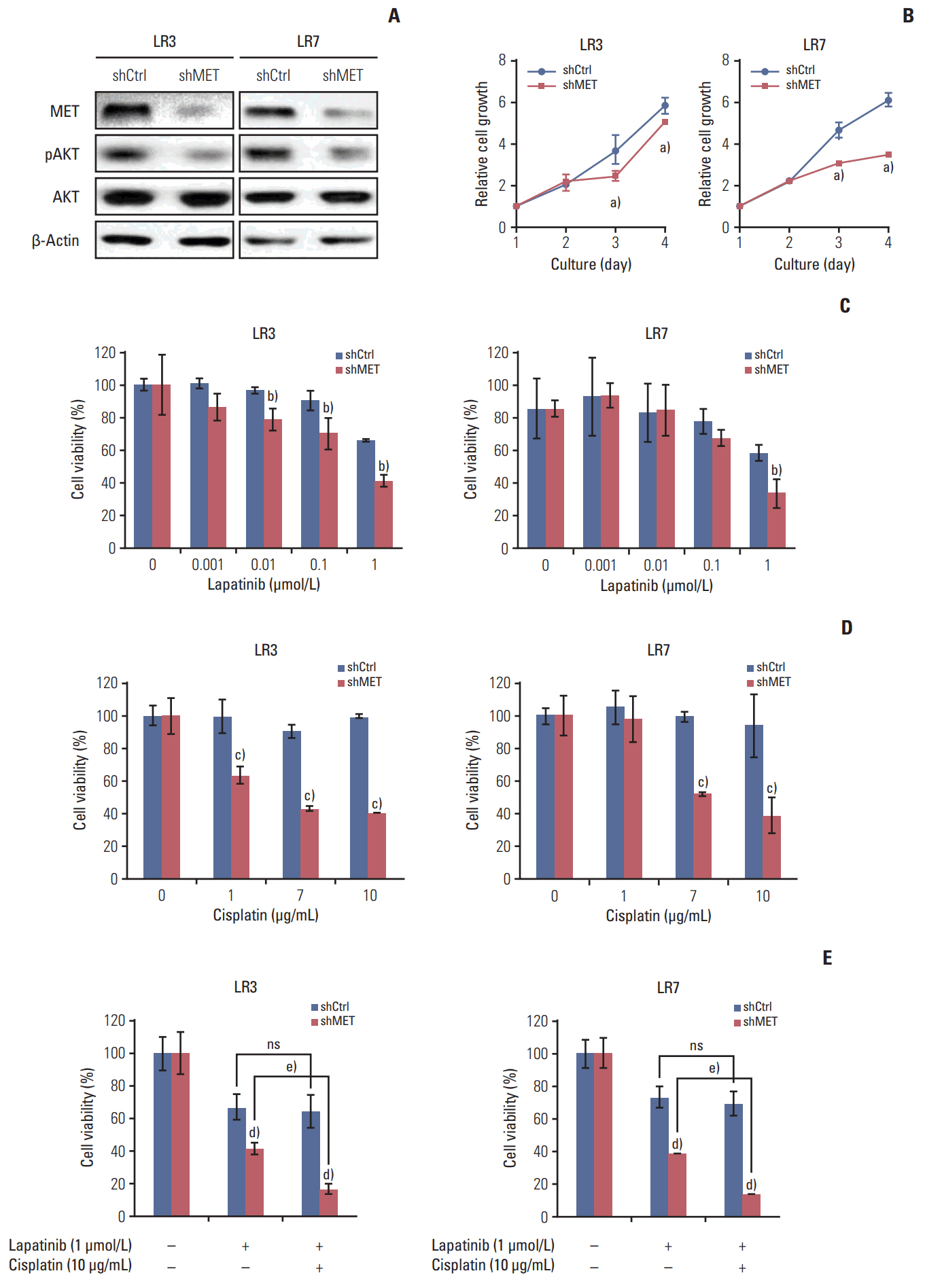
Lapatinib-resistant GC cell lines (SNU-216 LR2-8) were generated in vitro by chronic exposure of lapatinib-sensitive, HER2-positive SNU-216 cells to lapatinib. SNU-216 LR cells with FOXO1 overexpression were generated by stable transfection of a constitutively active FOXO1 mutant (FOXO1A3). HER2 and MET in SNU-216 LR cells were downregulated using RNA interference. The sensitivity of GC cells to lapatinib and/or cisplatin was determined by crystal violet assay. In addition, Western blot analysis, luciferase reporter assay and reverse transcription-polymerase chain reaction were performed.
RESULTS
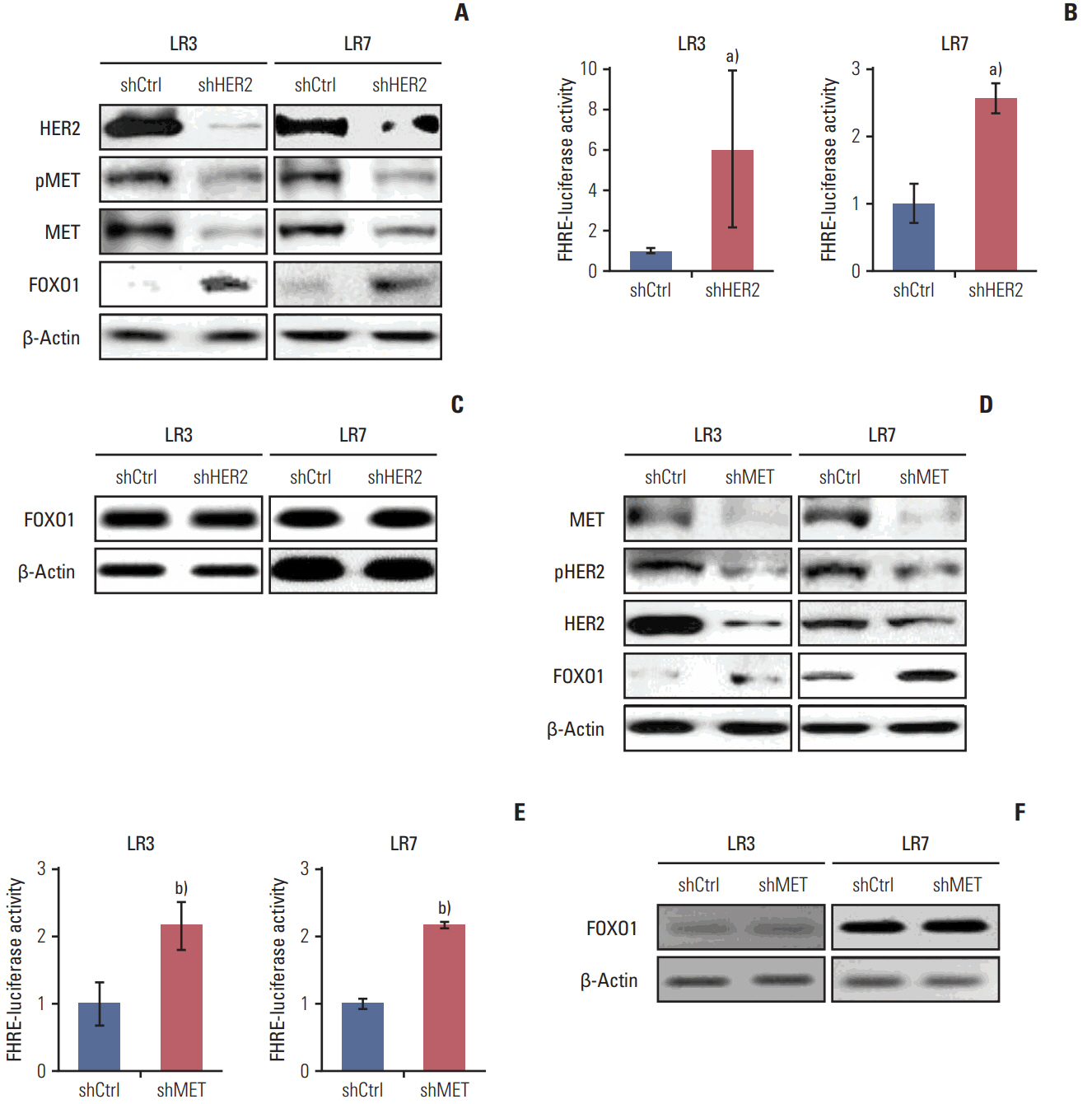
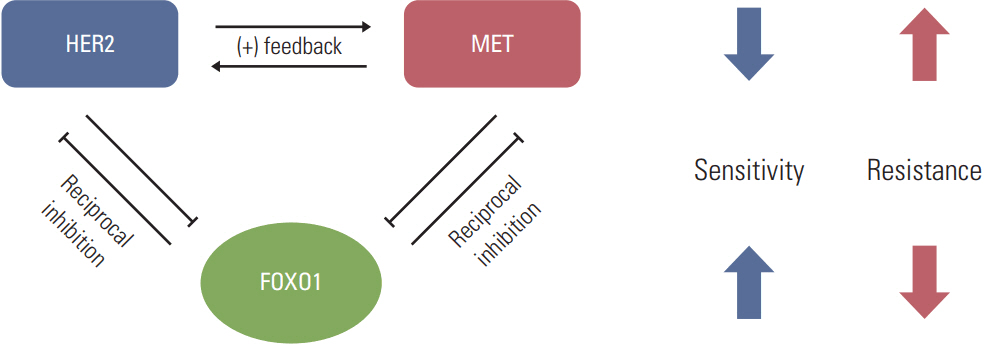
SNU-216 LR cells showed upregulations of HER2 and MET, but downregulation of FOXO1 compared to parental SNU-216 cells. FOXO1 overexpression in SNU-216 LR cells significantly suppressed resistance to lapatinib and/or cisplatin. In addition, FOXO1 negatively controlled HER2 and MET at the transcriptional level and was negatively controlled by these molecules at the post-transcriptional level. A positive crosstalk was shown between HER2 and MET, each of which increased resistance to lapatinib and/or cisplatin.
CONCLUSION
FOXO1 serves as an important linker between HER2 and MET signaling pathways through negative crosstalks and is a key regulator of the acquired lapatinib resistance in HER2-positive GC cells. These findings provide a rationale for establishing a novel treatment strategy to overcome lapatinib resistance in a subtype of GC patients.
Keyword
MeSH Terms
-
Blotting, Western
Cell Line
Cisplatin
Down-Regulation
Drug Resistance
Gentian Violet
Humans
In Vitro Techniques
Luciferases
Parents
Receptor, Epidermal Growth Factor
Receptor, ErbB-2
RNA Interference
Stomach Neoplasms*
Transfection
Up-Regulation*
Cisplatin
Gentian Violet
Luciferases
Receptor, Epidermal Growth Factor
Receptor, ErbB-2
Figure
Reference
-
References
1. Gravalos C, Jimeno A. HER2 in gastric cancer: a new prognostic factor and a novel therapeutic target. Ann Oncol. 2008; 19:1523–9.
Article2. Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010; 376:687–97.
Article3. Liu L, Wu N, Li J. Novel targeted agents for gastric cancer. J Hematol Oncol. 2012; 5:31.
Article4. Spector NL, Xia W, Burris H 3rd, Hurwitz H, Dees EC, Dowlati A, et al. Study of the biologic effects of lapatinib, a reversible inhibitor of ErbB1 and ErbB2 tyrosine kinases, on tumor growth and survival pathways in patients with advanced malignancies. J Clin Oncol. 2005; 23:2502–12.
Article5. Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007; 316:1039–43.6. Corso S, Ghiso E, Cepero V, Sierra JR, Migliore C, Bertotti A, et al. Activation of HER family members in gastric carcinoma cells mediates resistance to MET inhibition. Mol Cancer. 2010; 9:121.
Article7. Lee HE, Kim MA, Lee HS, Jung EJ, Yang HK, Lee BL, et al. MET in gastric carcinomas: comparison between protein expression and gene copy number and impact on clinical outcome. Br J Cancer. 2012; 107:325–33.
Article8. Nakajima M, Sawada H, Yamada Y, Watanabe A, Tatsumi M, Yamashita J, et al. The prognostic significance of amplification and overexpression of c-met and c-erb B-2 in human gastric carcinomas. Cancer. 1999; 85:1894–902.
Article9. Ha SY, Lee J, Jang J, Hong JY, Do IG, Park SH, et al. HER2-positive gastric cancer with concomitant MET and/or EGFR overexpression: a distinct subset of patients for dual inhibition therapy. Int J Cancer. 2015; 136:1629–35.
Article10. Chen CT, Kim H, Liska D, Gao S, Christensen JG, Weiser MR. MET activation mediates resistance to lapatinib inhibition of HER2-amplified gastric cancer cells. Mol Cancer Ther. 2012; 11:660–9.
Article11. Zhang Z, Wang J, Ji D, Wang C, Liu R, Wu Z, et al. Functional genetic approach identifies MET, HER3, IGF1R, INSR pathways as determinants of lapatinib unresponsiveness in HER2-positive gastric cancer. Clin Cancer Res. 2014; 20:4559–73.
Article12. Zhao M, Luo R, Liu Y, Gao L, Fu Z, Fu Q, et al. miR-3188 regulates nasopharyngeal carcinoma proliferation and chemosensitivity through a FOXO1-modulated positive feedback loop with mTOR-p-PI3K/AKT-c-JUN. Nat Commun. 2016; 7:11309.
Article13. Goto T, Takano M, Hirata J, Tsuda H. The involvement of FOXO1 in cytotoxic stress and drug-resistance induced by paclitaxel in ovarian cancers. Br J Cancer. 2008; 98:1068–75.
Article14. Han CY, Cho KB, Choi HS, Han HK, Kang KW. Role of FoxO1 activation in MDR1 expression in adriamycin-resistant breast cancer cells. Carcinogenesis. 2008; 29:1837–44.
Article15. Park J, Ko YS, Yoon J, Kim MA, Park JW, Kim WH, et al. The forkhead transcription factor FOXO1 mediates cisplatin resistance in gastric cancer cells by activating phosphoinositide 3-kinase/Akt pathway. Gastric Cancer. 2014; 17:423–30.
Article16. Ko YS, Cho SJ, Park J, Kim Y, Choi YJ, Pyo JS, et al. Loss of FOXO1 promotes gastric tumour growth and metastasis through upregulation of human epidermal growth factor receptor 2/neu expression. Br J Cancer. 2015; 113:1186–96.
Article17. Kim HP, Han SW, Song SH, Jeong EG, Lee MY, Hwang D, et al. Testican-1-mediated epithelial-mesenchymal transition signaling confers acquired resistance to lapatinib in HER2-positive gastric cancer. Oncogene. 2014; 33:3334–41.
Article18. Kim WH, Schnaper HW, Nomizu M, Yamada Y, Kleinman HK. Apoptosis in human fibrosarcoma cells is induced by a multimeric synthetic Tyr-Ile-Gly-Ser-Arg (YIGSR)-containing polypeptide from laminin. Cancer Res. 1994; 54:5005–10.19. Kim JW, Kim HP, Im SA, Kang S, Hur HS, Yoon YK, et al. The growth inhibitory effect of lapatinib, a dual inhibitor of EGFR and HER2 tyrosine kinase, in gastric cancer cell lines. Cancer Lett. 2008; 272:296–306.
Article20. Satoh T, Xu RH, Chung HC, Sun GP, Doi T, Xu JM, et al. Lapatinib plus paclitaxel versus paclitaxel alone in the second-line treatment of HER2-amplified advanced gastric cancer in Asian populations: TyTAN: a randomized, phase III study. J Clin Oncol. 2014; 32:2039–49.21. Sebolt-Leopold JS, English JM. Mechanisms of drug inhibition of signalling molecules. Nature. 2006; 441:457–62.
Article22. Sharma SV, Settleman J. Oncogene addiction: setting the stage for molecularly targeted cancer therapy. Genes Dev. 2007; 21:3214–31.
Article23. Wu Y, Shang X, Sarkissyan M, Slamon D, Vadgama JV. FOXO1A is a target for HER2-overexpressing breast tumors. Cancer Res. 2010; 70:5475–85.
Article24. Chakrabarty A, Bhola NE, Sutton C, Ghosh R, Kuba MG, Dave B, et al. Trastuzumab-resistant cells rely on a HER2-PI3K-FoxO-survivin axis and are sensitive to PI3K inhibitors. Cancer Res. 2013; 73:1190–200.
Article25. Wang YC, Morrison G, Gillihan R, Guo J, Ward RM, Fu X, et al. Different mechanisms for resistance to trastuzumab versus lapatinib in HER2-positive breast cancers: role of estrogen receptor and HER2 reactivation. Breast Cancer Res. 2011; 13:R121.
Article26. Rusnak DW, Alligood KJ, Mullin RJ, Spehar GM, Arenas-Elliott C, Martin AM, et al. Assessment of epidermal growth factor receptor (EGFR, ErbB1) and HER2 (ErbB2) protein expression levels and response to lapatinib (Tykerb, GW57-2016) in an expanded panel of human normal and tumour cell lines. Cell Prolif. 2007; 40:580–94.
Article27. Huang D, Duan H, Huang H, Tong X, Han Y, Ru G, et al. Cisplatin resistance in gastric cancer cells is associated with HER2 upregulation-induced epithelial-mesenchymal transition. Sci Rep. 2016; 6:20502.
Article28. Karamouzis MV, Konstantinopoulos PA, Papavassiliou AG. Targeting MET as a strategy to overcome crosstalk-related resistance to EGFR inhibitors. Lancet Oncol. 2009; 10:709–17.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Phosphoproteomic analysis identifies activated MET-axis PI3K/AKT and MAPK/ERK in lapatinib-resistant cancer cell line
- Effective Treatment of Solitary Pituitary Metastasis with Panhypopituitarism in HER2-Positive Breast Cancer by Lapatinib
- Real-World Data of Pyrotinib-Based Therapy in Metastatic HER2-Positive Breast Cancer: Promising Efficacy in Lapatinib-Treated Patients and in Brain Metastasis
- Breakthroughs in the Systemic Treatment of HER2-Positive Advanced/Metastatic Gastric Cancer: From Singlet Chemotherapy to Triple Combination
- GASTric Cancer HER2 Re-Assessment Study 2 (GASTHER2): HER2 Re-assessment for Initially HER2-Negative Advanced Gastric Cancer Patients after Progression on First-Line Treatment