Cancer Res Treat.
2018 Jan;50(1):175-182. 10.4143/crt.2016.535.
Gemcitabine and Docetaxel Combination for Advanced Soft Tissue Sarcoma: A Nationwide Retrospective Study
- Affiliations
-
- 1Department of Pharmaceutical Management, Health Insurance Review & Assessment, Wonju, Korea.
- 2College of Pharmacy, Health, Social & Clinical Pharmacy, Chung-Ang University, Seoul, Korea. dongsuh75@gmail.com
- 3Division of Hematology-Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 4Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 5Division of Hematology and Medical Oncology, Department of Internal Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea.
- 6Division of Oncology/Hematology, Department of Internal Medicine, Korea University College of Medicine, Seoul, Korea. yhk0215@korea.ac.kr
- 7Cancer Deliberation Committee, Health Insurance Review & Assessment Service, Wonju, Korea.
- 8Department of Biostatistics, Korea University College of Medicine, Seoul, Korea.
- KMID: 2403487
- DOI: http://doi.org/10.4143/crt.2016.535
Abstract
- PURPOSE
This nationwide retrospective study was conducted to evaluate the efficacy and safety of combined gemcitabine and docetaxel (GD) as an off-label therapy for advanced soft tissue sarcoma, which has limited treatment options owing to its rare occurrence.
MATERIALS AND METHODS
A total of 228 patients received GD therapy for advanced soft tissue sarcoma from 2009 to 2014 in Korea. We retrospectively reviewed the clinical medical records and claims data of these patients.
RESULTS
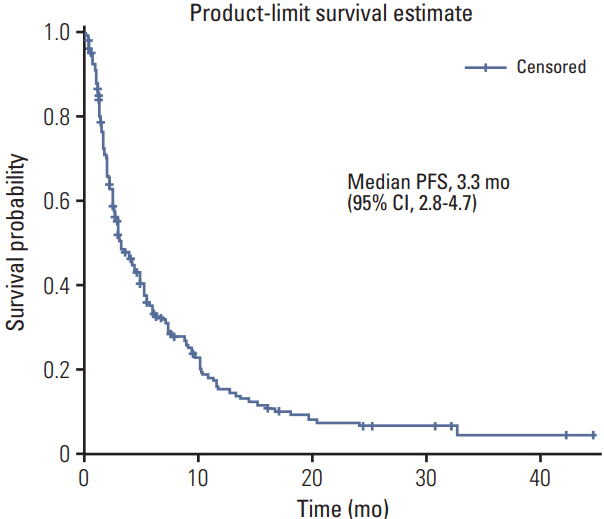
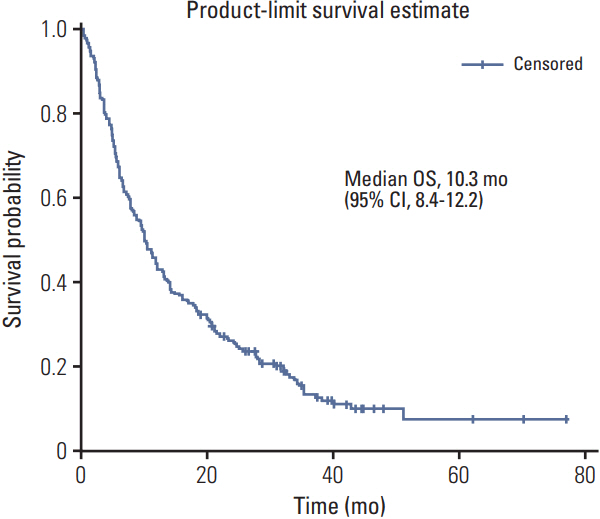
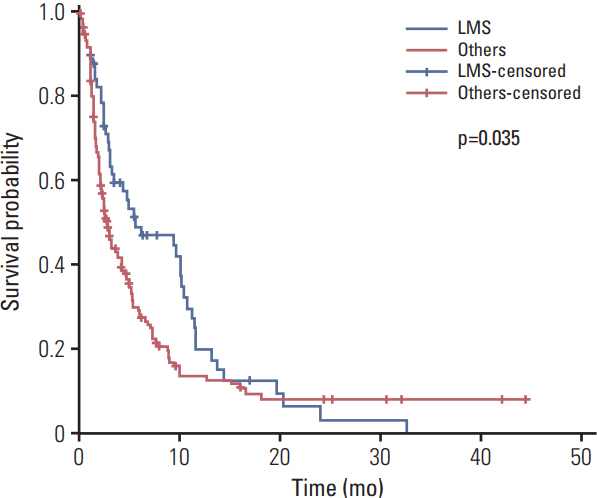
A total of 218 patients in 20 medical centers were included in the final analysis (median age, 50.0 years). The objective response rate was 15.1% (34/218, in the leiomyosarcoma subgroup; 26.3%). The median overall survival and progression-free survival were 10.3 months (95% confidence interval [CI], 8.4 to 12.2) and 3.3 months (95% CI, 2.8 to 4.7), respectively. The treatment was discontinued in 7.8% of patients owing to adverse events; however, there was no adverse event-related death. Neutropenia (35.7%) and anemia (15.1%) were the most frequent grade 3/4 toxicities. Univariate analysis for identifying the predictors of the progression-free survival period revealed that patients aged ≤ 50 years had a hazard ratio of 1.388 (95% CI, 1.027 to 1.875; p < 0.05) relative to those aged > 50 years, and the group with leiomyosarcoma had a hazard ratio of 0.693 (95% CI, 0.493 to 0.975; p < 0.05) relative to the group with other histopathological subtypes.
CONCLUSION
GD therapy was tolerable and effective for Korean patients with soft tissue sarcoma. In conclusion, for patients with advanced soft tissue sarcoma, especially leiomyosarcoma, GD therapy could be an important therapeutic option.
MeSH Terms
Figure
Reference
-
References
1. DeVita VT Jr, Lawrence TS, Rosenberg SA. DeVita, Hellman, and Rosenberg's cancer: principles and practice of oncology. 10th ed. Philadelphia, PA: Lippincott Williams & Wilkins;2014.2. Clark MA, Fisher C, Judson I, Thomas JM. Soft-tissue sarcomas in adults. N Engl J Med. 2005; 353:701–11.
Article3. Santoro A, Tursz T, Mouridsen H, Verweij J, Steward W, Somers R, et al. Doxorubicin versus CYVADIC versus doxorubicin plus ifosfamide in first-line treatment of advanced soft tissue sarcomas: a randomized study of the European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. J Clin Oncol. 1995; 13:1537–45.4. Elias A, Ryan L, Sulkes A, Collins J, Aisner J, Antman KH. Response to mesna, doxorubicin, ifosfamide, and dacarbazine in 108 patients with metastatic or unresectable sarcoma and no prior chemotherapy. J Clin Oncol. 1989; 7:1208–16.
Article5. Antman KH, Ryan L, Elias A, Sherman D, Grier HE. Response to ifosfamide and mesna: 124 previously treated patients with metastatic or unresectable sarcoma. J Clin Oncol. 1989; 7:126–31.
Article6. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN Guidelines): soft tissue sarcoma. Version 2 [Internet]. Fort Washington, PA: National Comprehensive Cancer Network;[cited 2017 Mar 2]. Available from: http://www.nccn.org/professionals/physician_gls/pdf/sarcoma.pdf.7. ESMO/European Sarcoma Network Working Group. Soft tissue and visceral sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014; 25 Suppl 3:iii102–12.8. Sheng JY, Movva S. Systemic therapy for advanced soft tissue sarcoma. Surg Clin North Am. 2016; 96:1141–56.
Article9. Ratan R, Patel SR. Chemotherapy for soft tissue sarcoma. Cancer. 2016; 122:2952–60.
Article10. Colosia A, Khan S, Hackshaw MD, Oglesby A, Kaye JA, Skolnik JM. A systematic literature review of adverse events associated with systemic treatments used in advanced soft tissue sarcoma. Sarcoma. 2016; 2016:3597609.
Article11. Bay JO, Ray-Coquard I, Fayette J, Leyvraz S, Cherix S, Piperno-Neumann S, et al. Docetaxel and gemcitabine combination in 133 advanced soft-tissue sarcomas: a retrospective analysis. Int J Cancer. 2006; 119:706–11.
Article12. Maki RG, Wathen JK, Patel SR, Priebat DA, Okuno SH, Samuels B, et al. Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: results of sarcoma alliance for research through collaboration study 002. J Clin Oncol. 2007; 25:2755–63.
Article13. Kaya AO, Buyukberber S, Ozkan M, Alkis N, Sevinc A, Ozdemir NY, et al. Efficacy and toxicity of gemcitabine plus docetaxel combination as a second line therapy for patients with advanced stage soft tissue sarcoma. Asian Pac J Cancer Prev. 2012; 13:463–7.14. Pink D, Richter S, Gerdes S, Andreou D, Tunn PU, Busemann C, et al. Gemcitabine and docetaxel for epithelioid sarcoma: results from a retrospective, multi-institutional analysis. Oncology. 2014; 87:95–103.
Article15. Rapkin L, Qayed M, Brill P, Martin M, Clark D, George BA, et al. Gemcitabine and docetaxel (GEMDOX) for the treatment of relapsed and refractory pediatric sarcomas. Pediatr Blood Cancer. 2012; 59:854–8.
Article16. Schmitt T, Kosely F, Wuchter P, Schmier JW, Ho AD, Egerer G. Gemcitabine and docetaxel for metastatic soft tissue sarcoma: a single center experience. Onkologie. 2013; 36:415–20.17. Lee EM, Rha SY, Lee J, Park KH, Ahn JH. Phase II study of weekly docetaxel and fixed dose rate gemcitabine in patients with previously treated advanced soft tissue and bone sarcoma. Cancer Chemother Pharmacol. 2012; 69:635–42.
Article18. Lee HY, Shin SJ, Kim HS, Hong SJ, Han JW, Lim ST, et al. Weekly gemcitabine and docetaxel in refractory soft tissue sarcoma: a retrospective analysis. Cancer Res Treat. 2012; 44:43–9.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Weekly Gemcitabine and Docetaxel in Refractory Soft Tissue Sarcoma: A Retrospective Analysis
- Comparative Evaluation of Second-Line Chemotherapy Agents for Advanced Soft Tissue Sarcoma: Gemcitabine/Docetaxel, Pazopanib, and Alternatives
- Alveolar soft part Sarcoma with Metastasis to Bone: A Case Report
- Chemotherapy of Advanced Soft Tissue Sarcoma with Etoposide, Ifosfamide, and Cisplatin (VIP)
- Combination chemotherapy with docetaxel and cisplatin as first-line treatment in advanced gastric cancer: is it a new effective chemotherapy?