Ann Lab Med.
2018 May;38(3):204-211. 10.3343/alm.2018.38.3.204.
Prognostic Role of High-sensitivity Cardiac Troponin I and Soluble Suppression of Tumorigenicity-2 in Surgical Intensive Care Unit Patients Undergoing Non-cardiac Surgery
- Affiliations
-
- 1Department of Cardiovascular Medicine, Konkuk University School of Medicine, Seoul, Korea.
- 2Department of Laboratory Medicine, Konkuk University School of Medicine, Seoul, Korea. dearmina@hanmail.net
- 3Research Coordinating Center, Konkuk University Medical Center, Seoul, Korea.
- KMID: 2403453
- DOI: http://doi.org/10.3343/alm.2018.38.3.204
Abstract
- BACKGROUND
The prognostic utility of cardiac biomarkers, high-sensitivity cardiac troponin I (hs-cTnI) and soluble suppression of tumorigenicity-2 (sST2), in non-cardiac surgery is not well-defined. We evaluated hs-cTnI and sST2 as predictors of 30-day major adverse cardiac events (MACE) in patients admitted to the surgical intensive care unit (SICU) following major non-cardiac surgery.
METHODS
hs-cTnI and sST2 concentrations were measured in 175 SICU patients immediately following surgery and for three days postoperatively. The results were analyzed in relation to 30-day MACE and were compared with the revised Goldman cardiac risk index (RCRI) score.
RESULTS
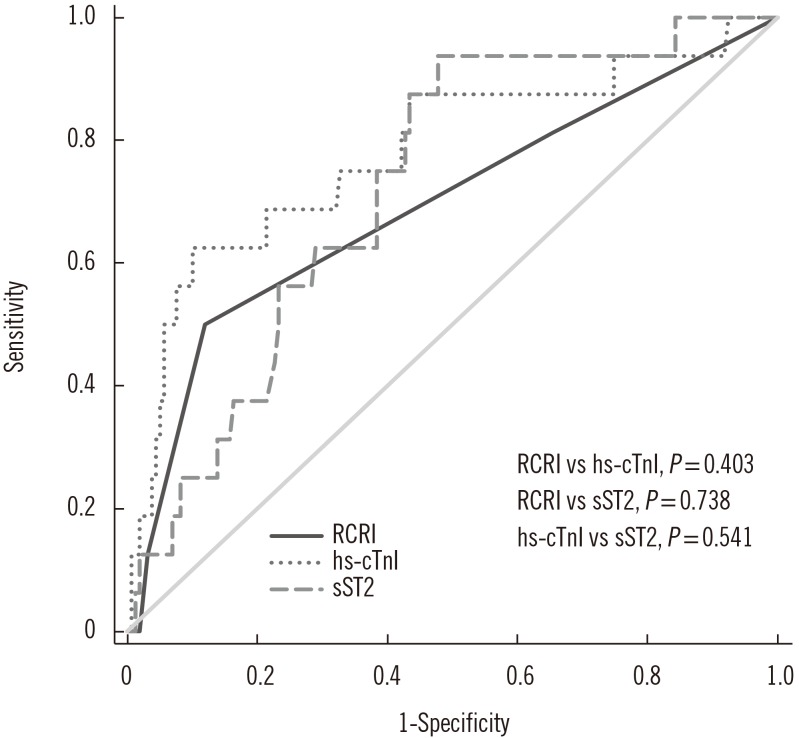
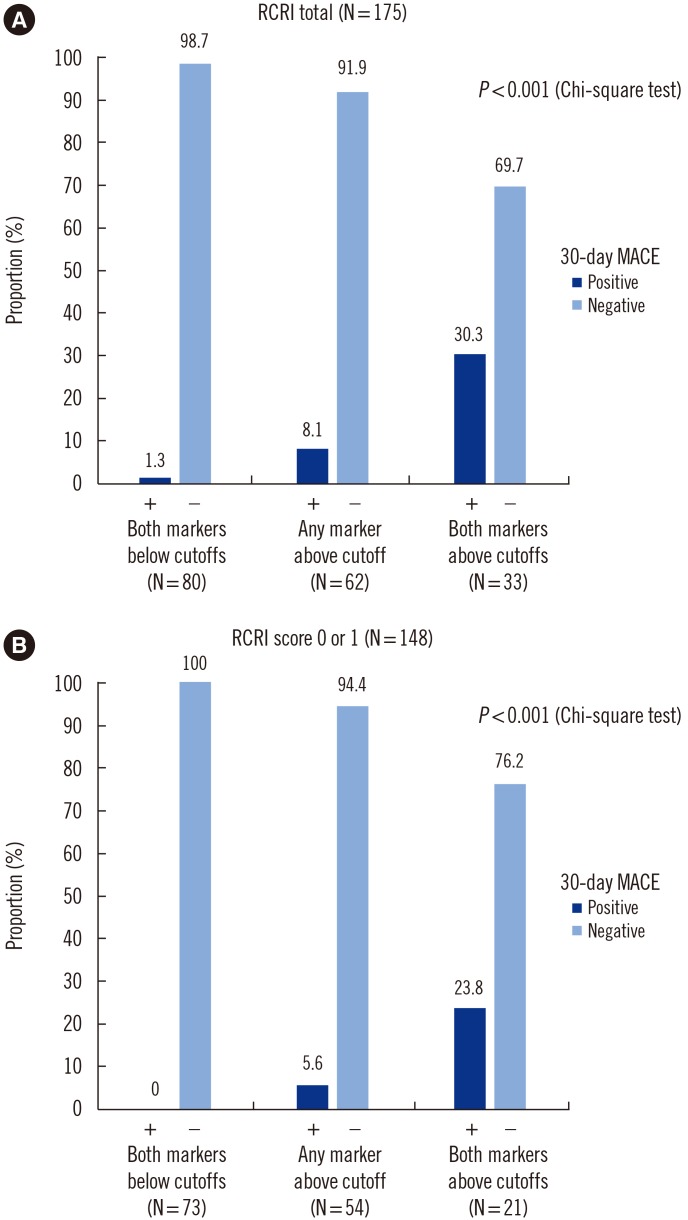
Overall, 30-day MACE was observed in 16 (9.1%) patients. hs-cTnI and sST2 concentrations differed significantly between the two groups with and without 30-day MACE (P < 0.05). The maximum concentration of sST2 was an independent predictor of 30-day MACE (odds ratio=1.016, P=0.008). The optimal cut-off values of hs-cTnI and sST2 for predicting 30-day MACE were 53.0 ng/L and 182.5 ng/mL, respectively. A combination of hs-cTnI and sST2 predicted 30-day MACE better than the RCRI score. Moreover, 30-day MACE was observed more frequently with increasing numbers of above-optimal cut-off hs-cTnI and sST2 values (P < 0.0001). Reclassification analyses indicated that the addition of biomarkers to RCRI scores improved the prediction of 30-day MACE.
CONCLUSIONS
This study demonstrates the utility of hs-cTnI and sST2 in predicting 30-day MACE following non-cardiac surgery. Cardiac biomarkers would provide enhanced risk stratification in addition to clinical RCRI scores for patients undergoing major non-cardiac surgery.
Keyword
Figure
Reference
-
1. McCarthy CP, van Kimmenade RRJ, Gaggin HK, Simon ML, Ibrahim NE, Gandhi P, et al. Usefulness of multiple biomarkers for predicting incident major adverse cardiac events in patients who underwent diagnostic coronary angiography (from the Catheter Sampled Blood Archive in Cardiovascular Disease [CASABLANCA] Study). Am J Cardiol. 2017; 120:25–32. PMID: 28487034.2. Kip KE, Hollabaugh K, Marroquin OC, Williams DO. The problem with composite end points in cardiovsacular studies. J Am Coll Cardiol. 2008; 51:701–707. PMID: 18279733.3. Khuri SF, Daley J, Henderson W, Hur K, Demakis J, Aust JB, et al. The Department of Veterans Affairs’ NSQIP: the first national, validated, outcome-based, risk-adjusted, and peer-controlled program for the measurement and enhancement of the quality of surgical care. National VA Surgical Quality Improvement Program. Ann Surg. 1998; 228:491–507. PMID: 9790339.4. Lee TH, Marcantonio ER, Mangione CM, Thomas EJ, Polanczyk CA, Cook EF, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999; 100:1043–1049. PMID: 10477528.5. Devereaux PJ, Goldman L, Cook DJ, Gilbert K, Leslie K, Guyatt GH. Perioperative cardiac events in patients undergoing noncardiac surgery: a review of the magnitude of the problem, the pathophysiology of the events and methods to estimate and communicate risk. CMAJ. 2005; 173:627–634. PMID: 16157727.6. Kristensen SD, Knuuti J, Saraste A, Anker S, Bøtker HE, Hert SD, et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: The Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J. 2014; 35:2383–2431. PMID: 25086026.7. Fleisher LA, Fleischmann KE, Auerbach AD, Barnason SA, Beckman JA, Bozkurt B, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2014; 64:e77–e137. PMID: 25091544.8. Ford MK, Beattie WS, Wijeysundera DN. Systematic review: prediction of perioperative cardiac complications and mortality by the revised cardiac risk index. Ann Intern Med. 2010; 152:26–35. PMID: 20048269.9. Botto F, Alonso-Coello P, Chan MT, Villar JC, Xavier D, Srinathan S, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology. 2014; 120:564–578. PMID: 24534856.10. Biccard BM, Scott DJA, Chan MTV, Archbold A, Wang CY, Sigamani A, et al. Myocardial injury after noncardiac surgery (MINS) in vascular surgical patients: a prospective observational cohort study. Ann Surg. 5. 8. DOI: 10.1097/SLA.0000000000002290. [Epub ahead of print].11. Vascular Events in Noncardiac Surgery Patients Cohort Evaluation (VISION) Study Investigators. Devereaux PJ, Chan MT, Alonso-Coello P, Walsh M, Berwanger O, et al. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA. 2012; 307:2295–2304. PMID: 22706835.12. Writing Committee for the VISION Study Investigators. Devereaux PJ, Biccard BM, Sigamani A, Xavier D, Chan MTV, et al. Association of postoperative high-sensitivity troponin levels with myocardial injury and 30-day mortality among patients undergoing noncardiac surgery. JAMA. 2017; 317:1642–1651. PMID: 28444280.13. Brown JC, Samaha E, Rao S, Helwani MA, Duma A, Brown F, et al. High-sensitivity cardiac Troponin T improves the diagnosis of perioperative MI. Anesth Analg. 2017; 125:1455–1462. PMID: 28719430.14. Aimo A, Vergaro G, Passino C, Ripoli A, Ky B, Miller WL, et al. Prognostic value of soluble suppression of tumorigenicity-2 in chronic heart failure: a meta-analysis. JACC Heart Fail. 2017; 5:280–286. PMID: 27816512.15. Yang HS, Kim HJ, Shim HJ, Kim SJ, Hur M, Di Somma S, et al. Soluble ST2 and troponin I combination: useful biomarker for predicting development of stress cardiomyopathy in patients admitted to the medical intensive care unit. Heart Lung. 2015; 44:282–288. PMID: 26077689.16. Barbarash O, Gruzdeva O, Uchasova E, Dyleva Y, Belik E, Akbasheva O, et al. Prognostic value of soluble ST2 during hospitalization for ST-segment elevation myocardial infarction. Ann Lab Med. 2016; 36:313–319. PMID: 27139603.17. Marino R, Magrini L, Orsini F, Russo V, Cardelli P, Salerno G, et al. Comparison between soluble ST2 and high-sensitivity Troponin I in predicting short-term mortality for patients presenting to the emergency department with chest pain. Ann Lab Med. 2017; 37:137–146. PMID: 28029000.18. Obokata M, Sunaga H, Ishida H, Ito K, Ogawa T, Ando Y, et al. Independent and incremental prognostic value of novel cardiac biomarkers in chronic hemodialysis patients. Am Heart J. 2016; 179:29–41. PMID: 27595677.19. Hur M, Kim H, Kim HJ, Yang HS, Magrini L, Marino R, et al. Soluble ST2 has a prognostic role in patients with suspected sepsis. Ann Lab Med. 2015; 35:570–577. PMID: 26354344.20. Mueller T, Dieplinger B. The Presage® ST2 Assay: analytical considerations and clinical applications for a high-sensitivity assay for measurement of soluble ST2. Expert Rev Mol Diagn. 2013; 13:13–30. PMID: 23256700.21. CLSI. User verification of performance for precision and true. 2nd ed. CLSI document EP15-A2. Wayne, PA, USA: Clinical and Laboratort Standards Institute;2005.22. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988; 44:837–845. PMID: 3203132.23. Pascual-Figal DA, Januzzi JL. The biology of ST2: the International ST2 Consensus Panel. Am J Cardiol. 2015; 115(7 Suppl):3B–7B.24. Torborg A, Ryan L, Kantor G, Biccard BM. The pharmacoeconomics of routine postoperative troponin surveillance to prevent and treat myocardial infarction after non-cardiac surgery. S Afr Med J. 2014; 104:619–623. PMID: 25212403.25. van Waes JA, Nathoe HM, de Graaff JC, Kemperman H, de Borst GJ, Peelen LM, et al. Myocardial injury after noncardiac surgery and its association with short-term mortality. Circulation. 2013; 127:2264–2271. PMID: 23667270.26. Apple FS, Sandoval Y, Jaffe AS, Ordonez-Llanos J. IFCC Task Force on Clinical Applications of Cardiac Bio-Markers. Cardiac Troponin Assays: guide to understanding analytical characteristics and their impact on clinical care. Clin Chem. 2017; 63:73–81. PMID: 28062612.27. Mangano DT. Adverse outcomes after surgery in the year 2001-a continuing odyssey. Anesthesiology. 1998; 88:561–564. PMID: 9523794.28. Lindenauer PK, Pekow P, Wang K, Mamidi DK, Gutierrez B, Benjamin EM. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med. 2005; 353:349–361. PMID: 16049209.29. Farzi S, Stojakovic T, Marko T, Sankin C, Rehak P, Gumpert R, et al. Role of N-terminal pro B-type natriuretic peptide in identifying patients at high risk for adverse outcome after emergent non-cardiac surgery. Br J Anaesth. 2013; 110:554–560. PMID: 23248094.30. Mauermann E, Puelacher C, Buse GL. Myocardial injury after noncardiac surgery: an underappreciated problem and current challenges. Curr Opin Anaesthesiol. 2016; 29:403–412. PMID: 27008065.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cardiac Complications in Patients Admitted to the Neuro-Intensive Care Unit
- Soluble Suppression of Tumorigenicity-2 as a Candidate Prognostic Marker for Stroke: A Systematic Review
- Are frailty scales better than anesthesia or surgical scales to determine risk in cardiac surgery?
- Performance Evaluation of the Point-of-Care Cardiac Troponin T Assay
- Management of Common Arrhythmia in the Neurological Intensive Care Unit