Cancer Res Treat.
2015 Oct;47(4):921-930. 10.4143/crt.2014.153.
Tumor Growth Suppression and Enhanced Radioresponse by an Exogenous Epidermal Growth Factor in Mouse Xenograft Models with A431 Cells
- Affiliations
-
- 1Department of Radiation Oncology, Seoul National University College of Medicine, Seoul, Korea. wuhg@snu.ac.kr
- 2Cancer Research Institution, Seoul National University College of Medicine, Seoul, Korea.
- 3Department of Pathology, Seoul National University College of Medicine, Seoul, Korea.
- 4Institute of Radiation Medicine, Medical Research Center, Seoul National University, Seoul, Korea.
- KMID: 2403413
- DOI: http://doi.org/10.4143/crt.2014.153
Abstract
- PURPOSE
The purpose of this study was to evaluate whether an exogenous epidermal growth factor (EGF) could induce anti-tumor and radiosensitizing effects in vivo.
MATERIALS AND METHODS
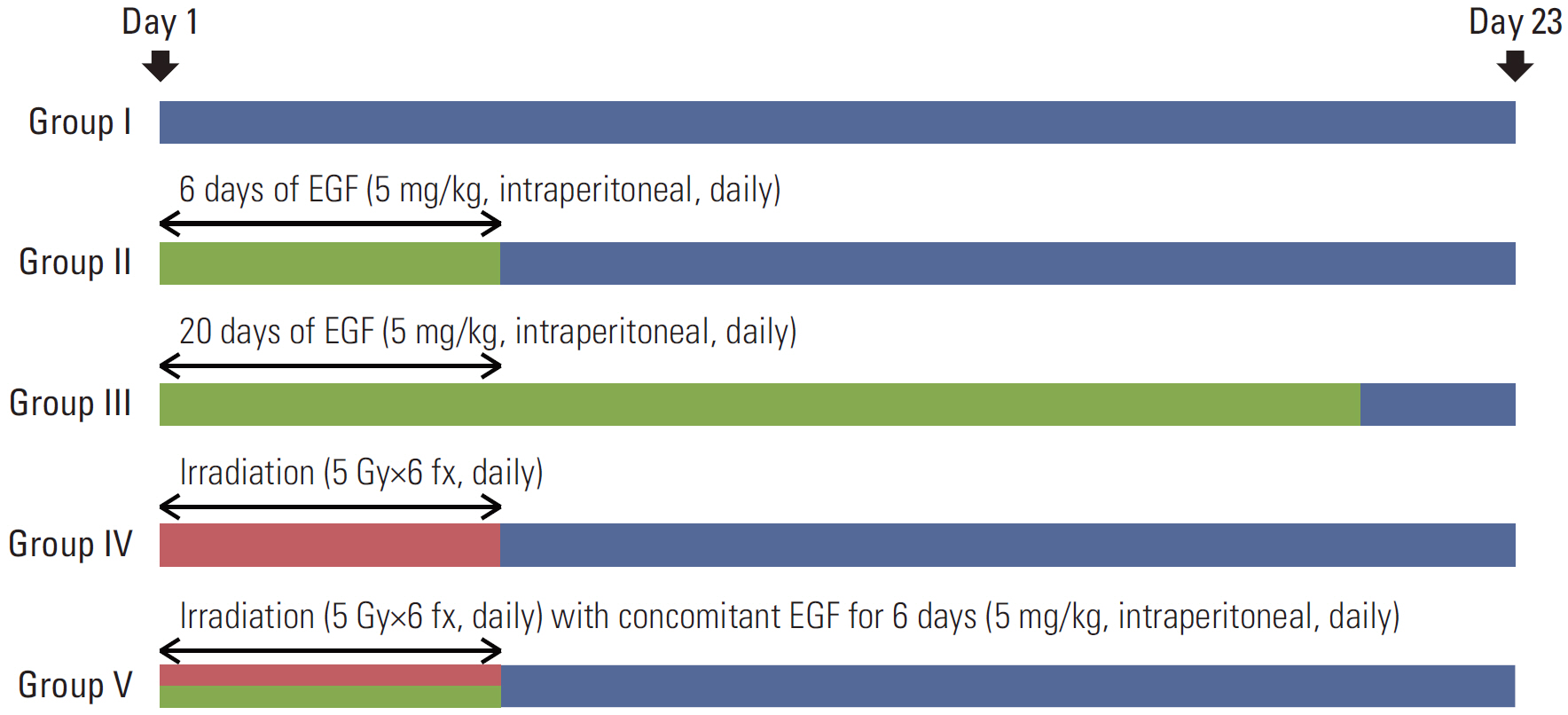
BALB/c-nu mice that were inoculated with A431 (human squamous cell carcinoma) cells in the right hind legs were divided into five groups: I (no treatment), II (EGF for 6 days), III (EGF for 20 days), IV (radiotherapy [RT]), and V (RT plus concomitant EGF). EGF was administered intraperitoneally (5 mg/kg) once a day and the RT dose was 30 Gy in six fractions. Hematoxylin and eosin (H&E) stained sections of tumor, liver, lung, and kidney tissues were investigated. Additionally, tumors were subjected to immunohistochemistry staining with caspase-3.
RESULTS
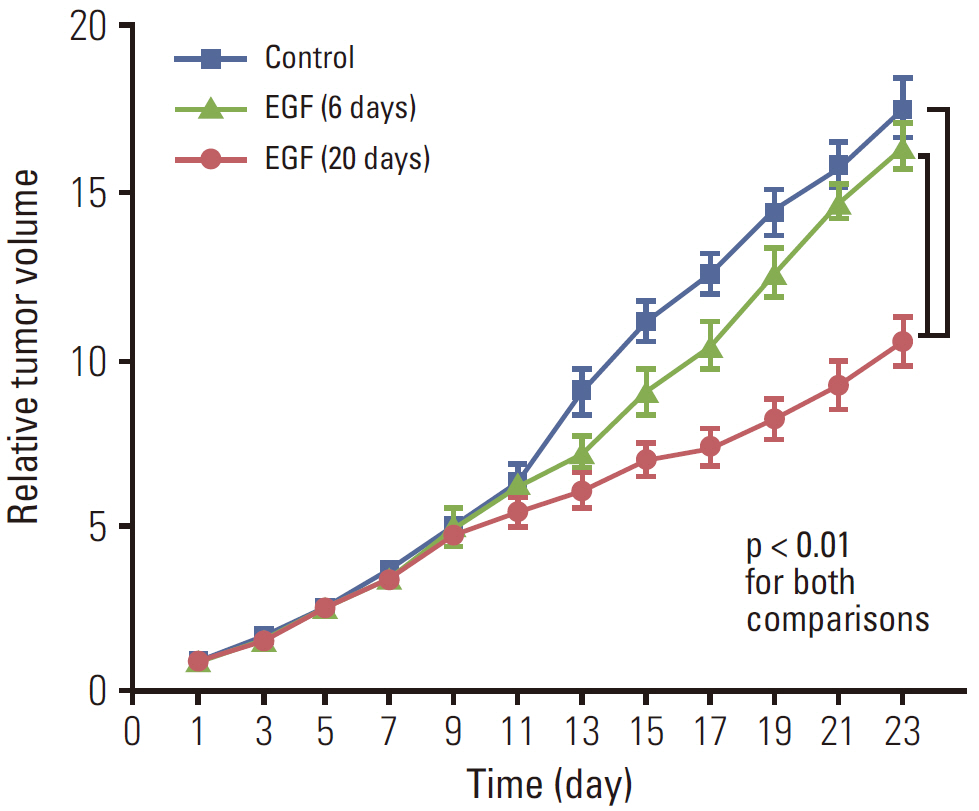
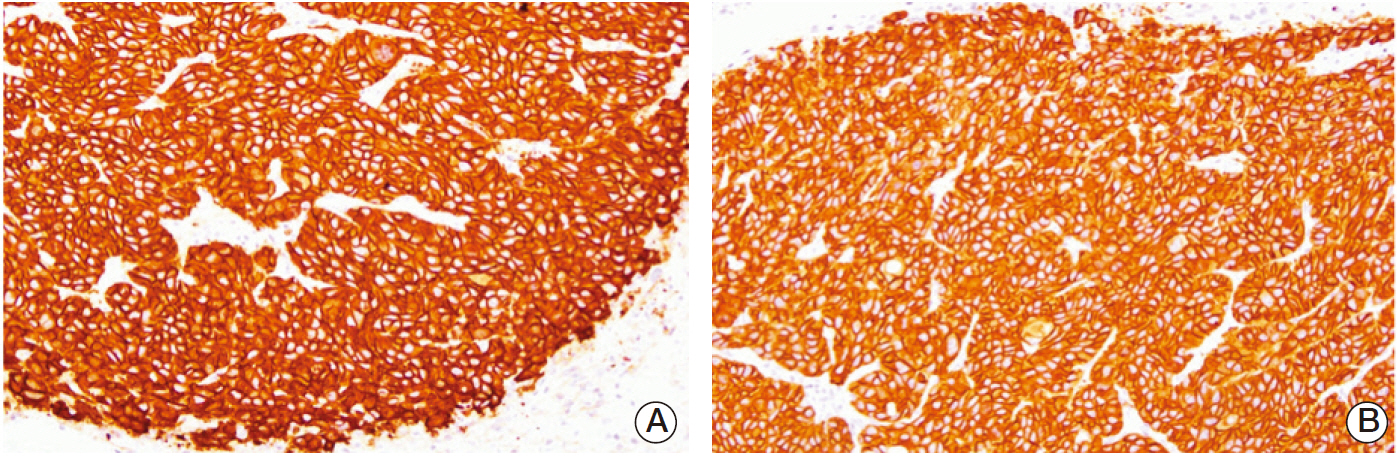
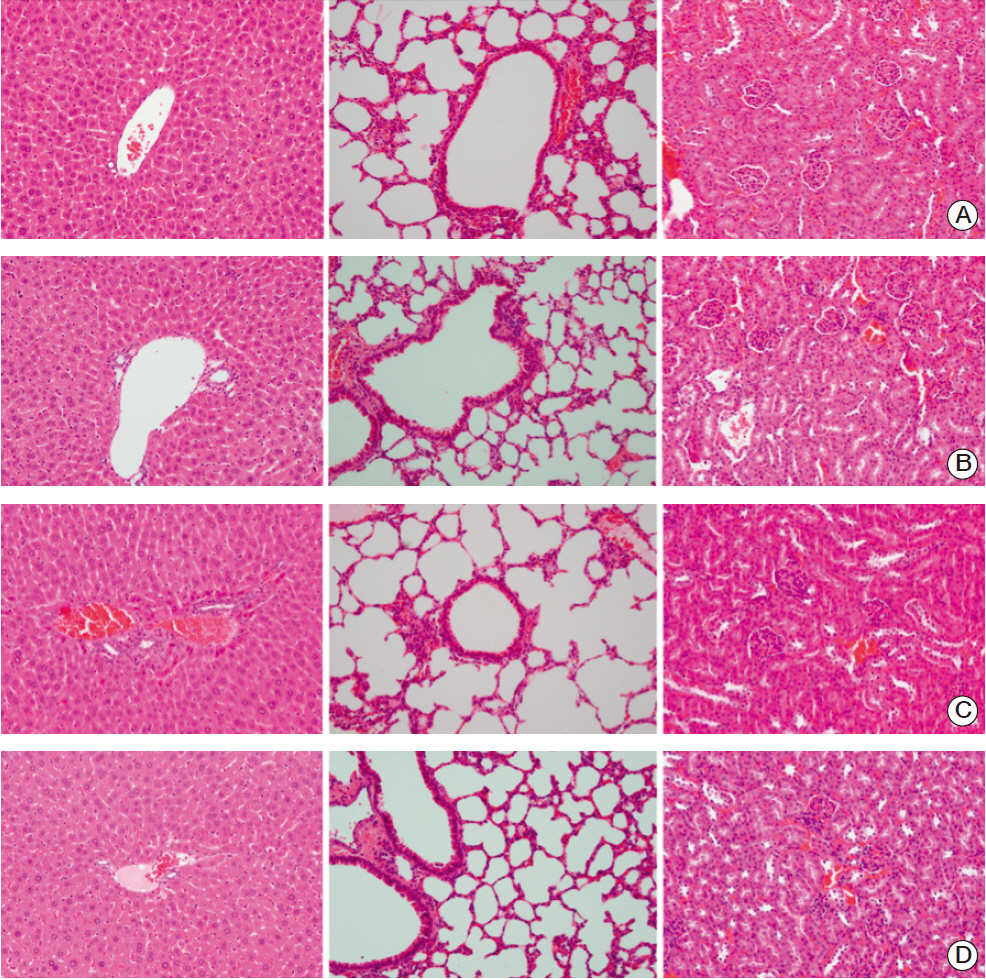
EGF for 6 days decreased tumor volume, but it approached the level of the control group at the end of follow-up (p=0.550). The duration of tumor shrinkage was prolonged in group V while the slope of tumor re-growth phase was steeper in group IV (p=0.034). EGF for 20 days decreased tumor volume until the end of the observation period (p < 0.001). Immunohistochemistry revealed that mice in group V showed stronger intensity than those in group IV. There were no abnormal histological findings upon H&E staining of the normal organs.
CONCLUSION
EGF-induced anti-tumor effect was ascertained in the xenograft mouse models with A431 cells. Concomitant use of EGF has the potential role as a radiosensitizer in the design of fractionated irradiation.
Keyword
MeSH Terms
-
Animals
Antineoplastic Agents
Apoptosis
Caspase 3
Eosine Yellowish-(YS)
Epidermal Growth Factor*
Follow-Up Studies
Hematoxylin
Heterografts*
Immunohistochemistry
Kidney
Leg
Liver
Lung
Mice*
Radiation-Sensitizing Agents
Tumor Burden
Xenograft Model Antitumor Assays
Antineoplastic Agents
Caspase 3
Eosine Yellowish-(YS)
Epidermal Growth Factor
Hematoxylin
Radiation-Sensitizing Agents
Figure
Reference
-
References
1. Boonstra J, Rijken P, Humbel B, Cremers F, Verkleij A, van Bergen en Henegouwen P. The epidermal growth factor. Cell Biol Int. 1995; 19:413–30.2. Henson ES, Gibson SB. Surviving cell death through epidermal growth factor (EGF) signal transduction pathways: implications for cancer therapy. Cell Signal. 2006; 18:2089–97.
Article3. Cao L, Yao Y, Lee V, Kiani C, Spaner D, Lin Z, et al. Epidermal growth factor induces cell cycle arrest and apoptosis of squamous carcinoma cells through reduction of cell adhesion. J Cell Biochem. 2000; 77:569–83.
Article4. Song JY, Lee SW, Hong JP, Chang SE, Choe H, Choi J. Epidermal growth factor competes with EGF receptor inhibitors to induce cell death in EGFR-overexpressing tumor cells. Cancer Lett. 2009; 283:135–42.
Article5. Worthylake R, Wiley HS. Structural aspects of the epidermal growth factor receptor required for transmodulation of erbB-2/neu. J Biol Chem. 1997; 272:8594–601.
Article6. Rush JS, Quinalty LM, Engelman L, Sherry DM, Ceresa BP. Endosomal accumulation of the activated epidermal growth factor receptor (EGFR) induces apoptosis. J Biol Chem. 2012; 287:712–22.
Article7. Kozyulina PY, Okorokova LS, Nikolsky NN, Grudinkin PS. p38 MAP kinase enhances EGF-induced apoptosis in A431 carcinoma cells by promoting tyrosine phosphorylation of STAT1. Biochem Biophys Res Commun. 2013; 430:331–5.
Article8. Grudinkin PS, Zenin VV, Kropotov AV, Dorosh VN, Nikolsky NN. EGF-induced apoptosis in A431 cells is dependent on STAT1, but not on STAT3. Eur J Cell Biol. 2007; 86:591–603.
Article9. Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. N Engl J Med. 2008; 358:1160–74.
Article10. Nyati MK, Morgan MA, Feng FY, Lawrence TS. Integration of EGFR inhibitors with radiochemotherapy. Nat Rev Cancer. 2006; 6:876–85.
Article11. Huang SM, Harari PM. Modulation of radiation response after epidermal growth factor receptor blockade in squamous cell carcinomas: inhibition of damage repair, cell cycle kinetics, and tumor angiogenesis. Clin Cancer Res. 2000; 6:2166–74.12. Bonner JA, Harari PM, Giralt J, Cohen RB, Jones CU, Sur RK, et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 2010; 11:21–8.
Article13. Kwok TT, Sutherland RM. Enhancement of sensitivity of human squamous carcinoma cells to radiation by epidermal growth factor. J Natl Cancer Inst. 1989; 81:1020–4.
Article14. Kwok TT, Sutherland RM. Differences in EGF related radiosensitisation of human squamous carcinoma cells with high and low numbers of EGF receptors. Br J Cancer. 1991; 64:251–4.
Article15. Bonner JA, Maihle NJ, Folven BR, Christianson TJ, Spain K. The interaction of epidermal growth factor and radiation in human head and neck squamous cell carcinoma cell lines with vastly different radiosensitivities. Int J Radiat Oncol Biol Phys. 1994; 29:243–7.
Article16. Licitra L, Perrone F, Tamborini E, Bertola L, Ghirelli C, Negri T, et al. Role of EGFR family receptors in proliferation of squamous carcinoma cells induced by wound healing fluids of head and neck cancer patients. Ann Oncol. 2011; 22:1886–93.
Article17. Choi J, Moon SY, Hong JP, Song JY, Oh KT, Lee SW. Epidermal growth factor induces cell death in the absence of overexpressed epidermal growth factor receptor and ErbB2 in various human cancer cell lines. Cancer Invest. 2010; 28:505–14.
Article18. Kwok TT, Sutherland RM. Cell cycle dependence of epidermal growth factor induced radiosensitization. Int J Radiat Oncol Biol Phys. 1992; 22:525–7.
Article19. Kwon EK, Lee SH, Kim K, Wu HG, Lee SW. Differential effects of recombinant human EGF on proliferation and radiation survival of normal fibroblast and cancer cell lines. Open Transl Med J. 2009; 1:9–15.
Article20. Chiu B, Mirkin B, Madonna MB. Novel action of epidermal growth factor on caspase 3 and its potential as a chemotherapeutic adjunct for neuroblastoma. J Pediatr Surg. 2007; 42:1389–95.
Article21. Fan Z, Lu Y, Wu X, DeBlasio A, Koff A, Mendelsohn J. Prolonged induction of p21Cip1/WAF1/CDK2/PCNA complex by epidermal growth factor receptor activation mediates ligand-induced A431 cell growth inhibition. J Cell Biol. 1995; 131:235–42.
Article22. Jakus J, Yeudall WA. Growth inhibitory concentrations of EGF induce p21 (WAF1/Cip1) and alter cell cycle control in squamous carcinoma cells. Oncogene. 1996; 12:2369–76.23. Bromberg JF, Fan Z, Brown C, Mendelsohn J, Darnell JE Jr. Epidermal growth factor-induced growth inhibition requires Stat1 activation. Cell Growth Differ. 1998; 9:505–12.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Epidermal growth factor receptors increase in rabbit embryonal implantation
- Amplification of epidermal growth factor receptor gene in primary cervical cancer
- Amplification of epidermal growth factor receptor gene in primary cervical cancer
- Study on the Effects of Epidermal Growth Factor on Epidermal and Hair Growth in the Mouse
- Correlation of epidermal growth factor receptor expression with prognostic factors in patients with ovarian neoplasms