Cancer Res Treat.
2015 Oct;47(4):853-861. 10.4143/crt.2014.177.
ATAD2 as a Poor Prognostic Marker for Hepatocellular Carcinoma after Curative Resection
- Affiliations
-
- 1Department of Pathology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. ckpark@skku.edu
- KMID: 2403405
- DOI: http://doi.org/10.4143/crt.2014.177
Abstract
- PURPOSE
Cancer cells frequently express genes that are specifically or preferentially expressed in male germ cells under normal conditions. The ATPase family AAA domain-containing 2 (ATAD2) is one such and works as an important cofactor for MYC-dependent transcription. In hepatocellular carcinoma (HCC), ATAD2 has been identified as a candidate driver gene located within the amplified 8q24 locus. However, the prognostic significance of ATAD2 protein expression in HCC remains uncertain.
MATERIALS AND METHODS
We investigated ATAD2 protein expression by immunohistochemistry in tumor tissue from 182 HCC patients who underwent curative resection. Associations of ATAD2 expression with clinicopathologic variables or prognosis of HCC patients were analyzed.
RESULTS
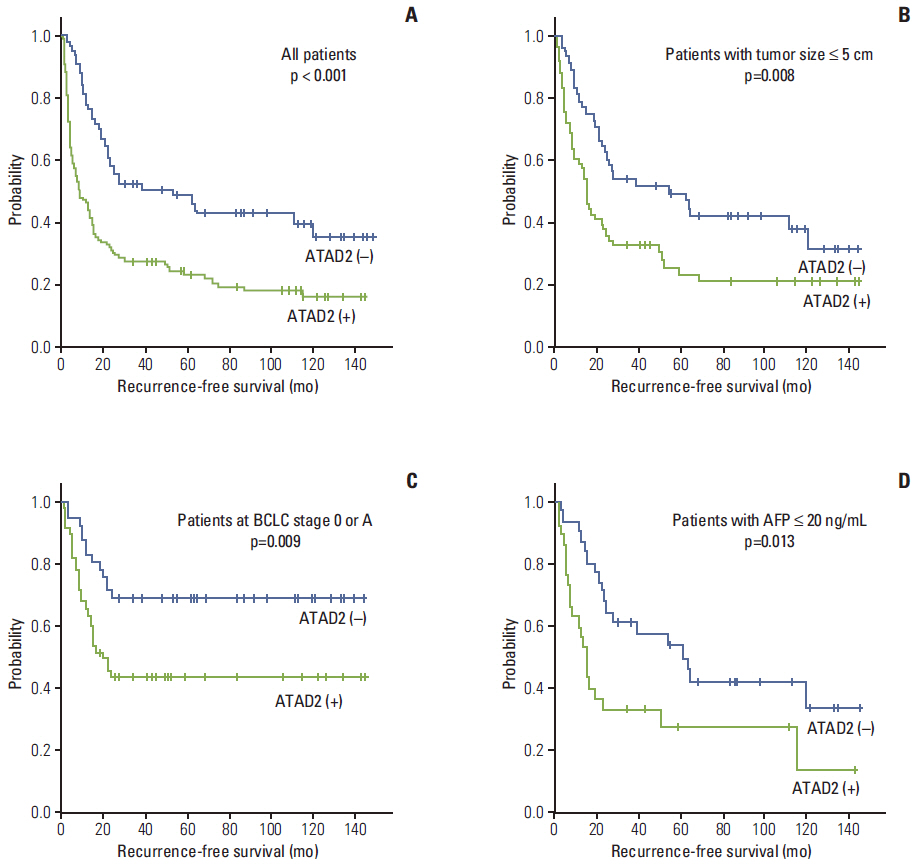
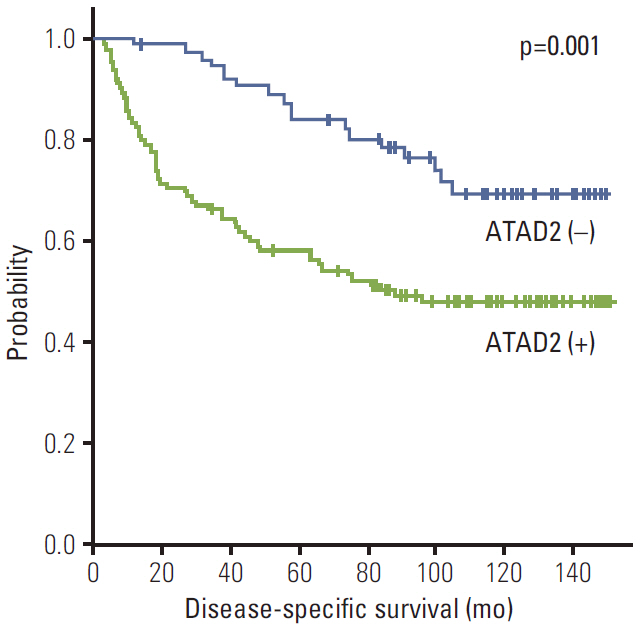
ATAD2 expression was observed in 119 (65.4%) of the 182 HCCs and tended to be independent predictor of early recurrence (p=0.059). ATAD2 expression showed an unfavorable influence on recurrence-free survival (RFS) (p < 0.001). Subgroup analysis among patients with tumor size < or = 5.0 cm (n=109), patients at Barcelona Clinic Liver Cancer stage 0 or A (n=92), and patients with alpha-fetoprotein < or = 20 ng/mL (n=61), the ATAD2-positive groups unfavorably influenced RFS (p=0.008, p=0.009, and p=0.013, respectively). In addition, ATAD2 expression was an independent predictor of shorter RFS (p=0.002). ATAD2 expression showed an unfavorable influence on disease-specific survival (p=0.001), but was not an independent predictor of shorter disease-specific survival (p=0.109).
CONCLUSION
ATAD2 protein expression may be a potential predictor of RFS in HCC patients after curative resection and ATAD2 may have prognostic value in patients with early stage HCC or normal serum alpha-fetoprotein level.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009; 27:1485–91.
Article2. Poon RT. Prevention of recurrence after resection of hepatocellular carcinoma: a daunting challenge. Hepatology. 2011; 54:757–9.
Article3. Qin LX, Tang ZY. Recent progress in predictive biomarkers for metastatic recurrence of human hepatocellular carcinoma: a review of the literature. J Cancer Res Clin Oncol. 2004; 130:497–513.
Article4. Rousseaux S, Khochbin S. New hypotheses for large-scale epigenome alterations in somatic cancer cells: a role for male germ-cell-specific regulators. Epigenomics. 2009; 1:153–61.
Article5. Ciro M, Prosperini E, Quarto M, Grazini U, Walfridsson J, McBlane F, et al. ATAD2 is a novel cofactor for MYC, overexpressed and amplified in aggressive tumors. Cancer Res. 2009; 69:8491–8.
Article6. Kalashnikova EV, Revenko AS, Gemo AT, Andrews NP, Tepper CG, Zou JX, et al. ANCCA/ATAD2 overexpression identifies breast cancer patients with poor prognosis, acting to drive proliferation and survival of triple-negative cells through control of B-Myb and EZH2. Cancer Res. 2010; 70:9402–12.
Article7. Caron C, Lestrat C, Marsal S, Escoffier E, Curtet S, Virolle V, et al. Functional characterization of ATAD2 as a new cancer/testis factor and a predictor of poor prognosis in breast and lung cancers. Oncogene. 2010; 29:5171–81.
Article8. Zou JX, Guo L, Revenko AS, Tepper CG, Gemo AT, Kung HJ, et al. Androgen-induced coactivator ANCCA mediates specific androgen receptor signaling in prostate cancer. Cancer Res. 2009; 69:3339–46.
Article9. Wang K, Lim HY, Shi S, Lee J, Deng S, Xie T, et al. Genomic landscape of copy number aberrations enables the identification of oncogenic drivers in hepatocellular carcinoma. Hepatology. 2013; 58:706–17.
Article10. Huang Q, Lin B, Liu H, Ma X, Mo F, Yu W, et al. RNA-Seq analyses generate comprehensive transcriptomic landscape and reveal complex transcript patterns in hepatocellular carcinoma. PLoS One. 2011; 6:e26168.
Article11. Wu G, Liu H, He H, Wang Y, Lu X, Yu Y, et al. miR-372 downregulates the oncogene ATAD2 to influence hepatocellular carcinoma proliferation and metastasis. BMC Cancer. 2014; 14:107.
Article12. Edmondson HA, Liu H, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer. 1954; 7:462–503.13. Kumada T, Nakano S, Takeda I, Sugiyama K, Osada T, Kiriyama S, et al. Patterns of recurrence after initial treatment in patients with small hepatocellular carcinoma. Hepatology. 1997; 25:87–92.
Article14. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. 7th ed. New York: Springer;2010.15. Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999; 19:329–38.
Article16. Shimada M, Hamatsu T, Yamashita Y, Rikimaru T, Taguchi K, Utsunomiya T, et al. Characteristics of multicentric hepatocellular carcinomas: comparison with intrahepatic metastasis. World J Surg. 2001; 25:991–5.
Article17. Imamura H, Matsuyama Y, Tanaka E, Ohkubo T, Hasegawa K, Miyagawa S, et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2003; 38:200–7.
Article18. Hoshida Y, Villanueva A, Kobayashi M, Peix J, Chiang DY, Camargo A, et al. Gene expression in fixed tissues and outcome in hepatocellular carcinoma. N Engl J Med. 2008; 35:1995–2004.
Article19. Ahn S, Hyeon J, Park CK. Metadherin is a prognostic predictor of hepatocellular carcinoma after curative hepatectomy. Gut Liver. 2013; 7:206–12.
Article20. Nagasue N. Liver resection for hepatocellular carcinoma: indications, techniques, complications, and prognostic factors. J Hepatobiliary Pancreat Surg. 1998; 5:7–13.
Article21. Cucchetti A, Piscaglia F, Caturelli E, Benvegnu L, Vivarelli M, Ercolani G, et al. Comparison of recurrence of hepatocellular carcinoma after resection in patients with cirrhosis to its occurrence in a surveilled cirrhotic population. Ann Surg Oncol. 2009; 16:413–22.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Two Cases of Early Recurred Hepatocellular Carcinoma after Surgical Resection Which Showed Different Outcomes
- Impact of prognostic nutritional index on the recurrence of hepatocellular carcinoma after a curative resection
- Prognosis after intrahepatic recurrence in the patients who underwent curative resection for hepatocellular carcinoma
- A Case of Long Term Survival in Patient with Early Intrahepatic Recurrence and Extrahepatic Metastasis after Curative Resection of Hepatocellular Carcinoma
- A Case of Rapidly Recurred Hepatocellular Carcinoma with Distant Metastasis after Surgical Resection