Cancer Res Treat.
2015 Oct;47(4):718-726. 10.4143/crt.2014.064.
Overexpression of Plasminogen Activator Inhibitor-1 in Advanced Gastric Cancer with Aggressive Lymph Node Metastasis
- Affiliations
-
- 1Department of Surgery, Seoul National University College of Medicine, Seoul, Korea. hkyang@snu.ac.kr
- 2Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea.
- 3Theragen Bio Institute, TheragenEtex, Suwon, Korea.
- 4Department of Surgery, SMG-SNU Boramae Medical Center, Seoul, Korea.
- 5Department of Pathology, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2403390
- DOI: http://doi.org/10.4143/crt.2014.064
Abstract
- PURPOSE
The purpose of this study is to investigate differentially expressed genes using DNA microarray between advanced gastric cancer (AGC) with aggressive lymph node (LN) metastasis and that with a more advanced tumor stage but without LN metastasis.
MATERIALS AND METHODS
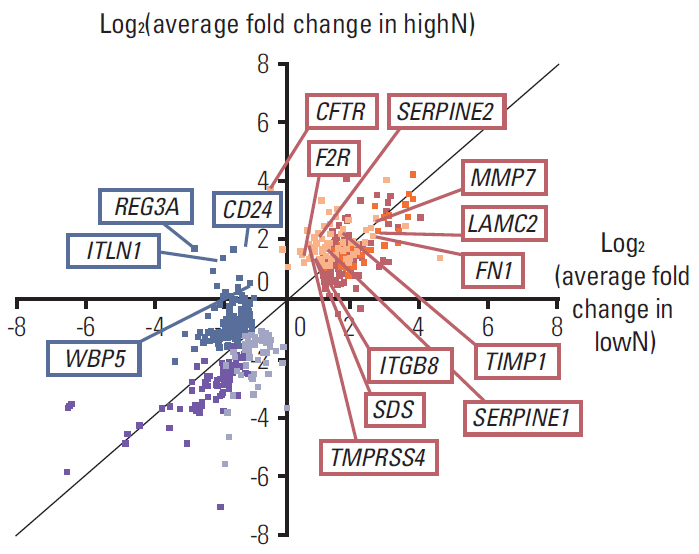
Five sample pairs of gastric cancer tissue and normal gastric mucosa were taken from three patients with T3N3 stage (highN) and two with T4N0 stage (lowN). Data from triplicate DNA microarray experiments were analyzed, and candidate genes were identified using a volcano plot that showed > or = 2-fold differential expression and were significant by Welch's t test (p < 0.05) between highN and lowN. Those selected genes were validated independently by reverse-transcriptase-polymerase chain reaction (RT-PCR) using five AGC patients, and tissue-microarray (TMA) comprising 47 AGC patients.
RESULTS
CFTR, LAMC2, SERPINE2, F2R, MMP7, FN1, TIMP1, plasminogen activator inhibitor-1 (PAI-1), ITGB8, SDS, and TMPRSS4 were commonly up-regulated over 2-fold in highN. REG3A, CD24, ITLN1, and WBP5 were commonly down-regulated over 2-fold in lowN. Among these genes, overexpression of PAI-1 was validated by RT-PCR, and TMA showed 16.7% (7/42) PAI-1 expression in T3N3, but none (0/5) in T4N0 (p=0.393).
CONCLUSION
DNA microarray analysis and validation by RT-PCR and TMA showed that overexpression of PAI-1 is related to aggressive LN metastasis in AGC.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010; 127:2893–917.
Article2. Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. 7th ed. New York: Springer;2010.3. Han DS, Suh YS, Kong SH, Lee HJ, Choi Y, Aikou S, et al. Nomogram predicting long-term survival after d2 gastrectomy for gastric cancer. J Clin Oncol. 2012; 30:3834–40.
Article4. Kampschoer GH, Maruyama K, van de Velde CJ, Sasako M, Kinoshita T, Okabayashi K. Computer analysis in making preoperative decisions: a rational approach to lymph node dissection in gastric cancer patients. Br J Surg. 1989; 76:905–8.5. Sasako M, McCulloch P, Kinoshita T, Maruyama K. New method to evaluate the therapeutic value of lymph node dissection for gastric cancer. Br J Surg. 1995; 82:346–51.
Article6. Weiss MM, Kuipers EJ, Postma C, Snijders AM, Siccama I, Pinkel D, et al. Genomic profiling of gastric cancer predicts lymph node status and survival. Oncogene. 2003; 22:1872–9.
Article7. Li W, Ye F, Wang D, Sun X, Tong W, Lian G, et al. Protein predictive signatures for lymph node metastasis of gastric cancer. Int J Cancer. 2013; 132:1851–9.
Article8. Marchet A, Mocellin S, Belluco C, Ambrosi A, DeMarchi F, Mammano E, et al. Gene expression profile of primary gastric cancer: towards the prediction of lymph node status. Ann Surg Oncol. 2007; 14:1058–64.
Article9. Suh YS, Lee HJ, Jung EJ, Kim MA, Nam KT, Goldenring JR, et al. The combined expression of metaplasia biomarkers predicts the prognosis of gastric cancer. Ann Surg Oncol. 2012; 19:1240–9.
Article10. Moussa A, Vannier B, Maouene M. New algorithm for gene selection in microarray data analysis workflow. Comput Technol Appl. 2012; 3:169–74.11. Golub TR, Slonim DK, Tamayo P, Huard C, Gaasenbeek M, Mesirov JP, et al. Molecular classification of cancer: class discovery and class prediction by gene expression monitoring. Science. 1999; 286:531–7.
Article12. Jin W, Riley RM, Wolfinger RD, White KP, Passador-Gurgel G, Gibson G. The contributions of sex, genotype and age to transcriptional variance in Drosophila melanogaster. Nat Genet. 2001; 29:389–95.
Article13. Li W, Freudenberg J, Suh YJ, Yang Y. Using volcano plots and regularized-chi statistics in genetic association studies. Comput Biol Chem. 2014; 48:77–83.
Article14. Dass K, Ahmad A, Azmi AS, Sarkar SH, Sarkar FH. Evolving role of uPA/uPAR system in human cancers. Cancer Treat Rev. 2008; 34:122–36.
Article15. de Bock CE, Wang Y. Clinical significance of urokinase-type plasminogen activator receptor (uPAR) expression in cancer. Med Res Rev. 2004; 24:13–39.
Article16. Andreasen PA, Kjoller L, Christensen L, Duffy MJ. The urokinase-type plasminogen activator system in cancer metastasis: a review. Int J Cancer. 1997; 72:1–22.
Article17. Bajou K, Maillard C, Jost M, Lijnen RH, Gils A, Declerck P, et al. Host-derived plasminogen activator inhibitor-1 (PAI-1) concentration is critical for in vivo tumoral angiogenesis and growth. Oncogene. 2004; 23:6986–90.
Article18. Nekarda H, Schmitt M, Ulm K, Wenninger A, Vogelsang H, Becker K, et al. Prognostic impact of urokinase-type plasminogen activator and its inhibitor PAI-1 in completely resected gastric cancer. Cancer Res. 1994; 54:2900–7.19. Sakakibara T, Hibi K, Koike M, Fujiwara M, Kodera Y, Ito K, et al. Plasminogen activator inhibitor-1 as a potential marker for the malignancy of gastric cancer. Cancer Sci. 2006; 97:395–9.
Article20. Ding Y, Zhang H, Zhong M, Zhou Z, Zhuang Z, Yin H, et al. Clinical significance of the uPA system in gastric cancer with peritoneal metastasis. Eur J Med Res. 2013; 18:28.
Article21. Nishioka N, Matsuoka T, Yashiro M, Hirakawa K, Olden K, Roberts JD. Plasminogen activator inhibitor 1 RNAi suppresses gastric cancer metastasis in vivo. Cancer Sci. 2012; 103:228–32.
Article22. Xie C, Jiang XH, Zhang JT, Sun TT, Dong JD, Sanders AJ, et al. CFTR suppresses tumor progression through miR-193b targeting urokinase plasminogen activator (uPA) in prostate cancer. Oncogene. 2013; 32:2282–91.23. Weis VG, Sousa JF, LaFleur BJ, Nam KT, Weis JA, Finke PE, et al. Heterogeneity in mouse spasmolytic polypeptide-expressing metaplasia lineages identifies markers of metaplastic progression. Gut. 2013; 62:1270–9.
Article24. Ahn HS, Lee HJ, Hahn S, Kim WH, Lee KU, Sano T, et al. Evaluation of the seventh American Joint Committee on Cancer/International Union Against Cancer Classification of gastric adenocarcinoma in comparison with the sixth classification. Cancer. 2010; 116:5592–8.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Plasminogen Activator Inhibitor-1, c-erbB2, and p53 Protein Overexpression and Prognosis in Gastric Adenocarcinoma
- Expression of urokinase: type plasminogen activator, its receptor, and its inhibitor in gastric adenocarcinoma tissues
- Expression of Urokinase-type Plasminogen Activator (uPA) and Plasminogen Activator Inhibitor-1 (PAI-1) in Gallbladder Carcinoma
- Expression of Matrix-Metalloproteinase-2, Urokinase-Type Plasminogen Activator and Plasminogen Activator Inhibitor-1 in Invasion Mode and Lymph Node Metastasis of Laryngeal Squamous Cell Carcinoma
- High Expression of Urokinase-Type Plasminogen Activator Is Associated with Lymph Node Metastasis of Invasive Ductal Carcinoma of the Breast