J Pathol Transl Med.
2018 Jan;52(1):51-55. 10.4132/jptm.2017.01.23.
An Autopsy Case of Epstein-Barr Virus–Associated Diffuse Large B-Cell Lymphoma of the Central Nervous System in an Immunocompromised Host
- Affiliations
-
- 1Department of Pathology, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2403258
- DOI: http://doi.org/10.4132/jptm.2017.01.23
Abstract
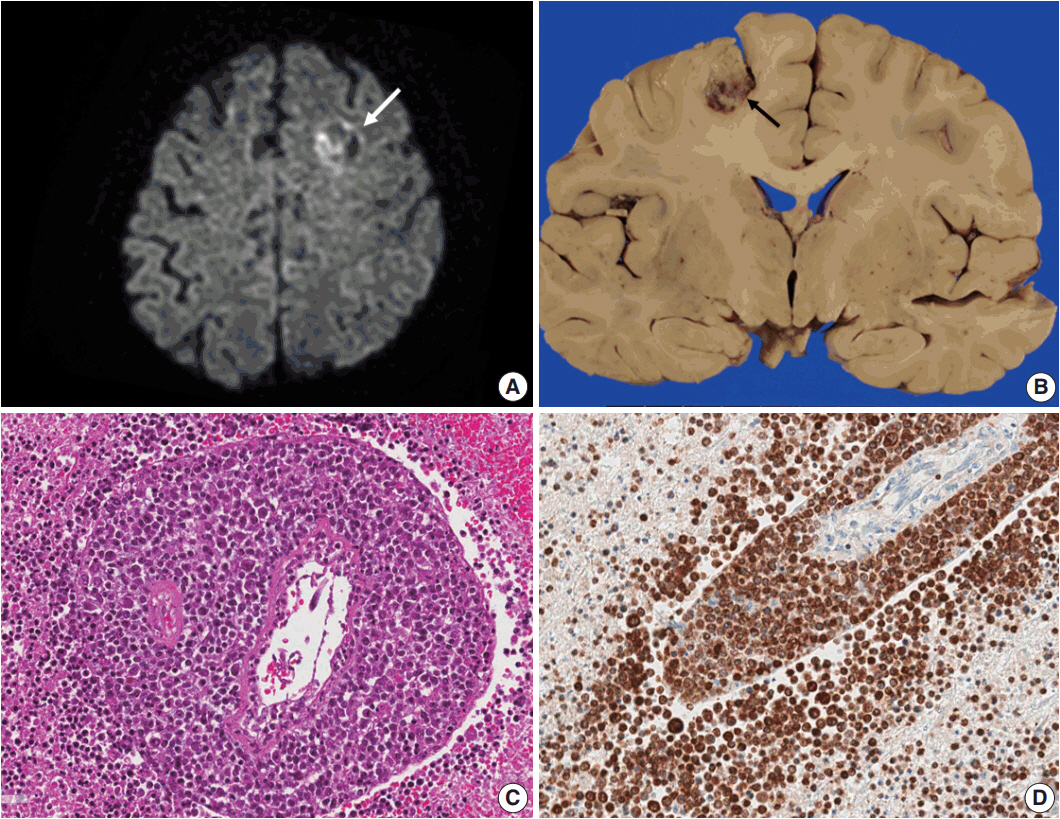
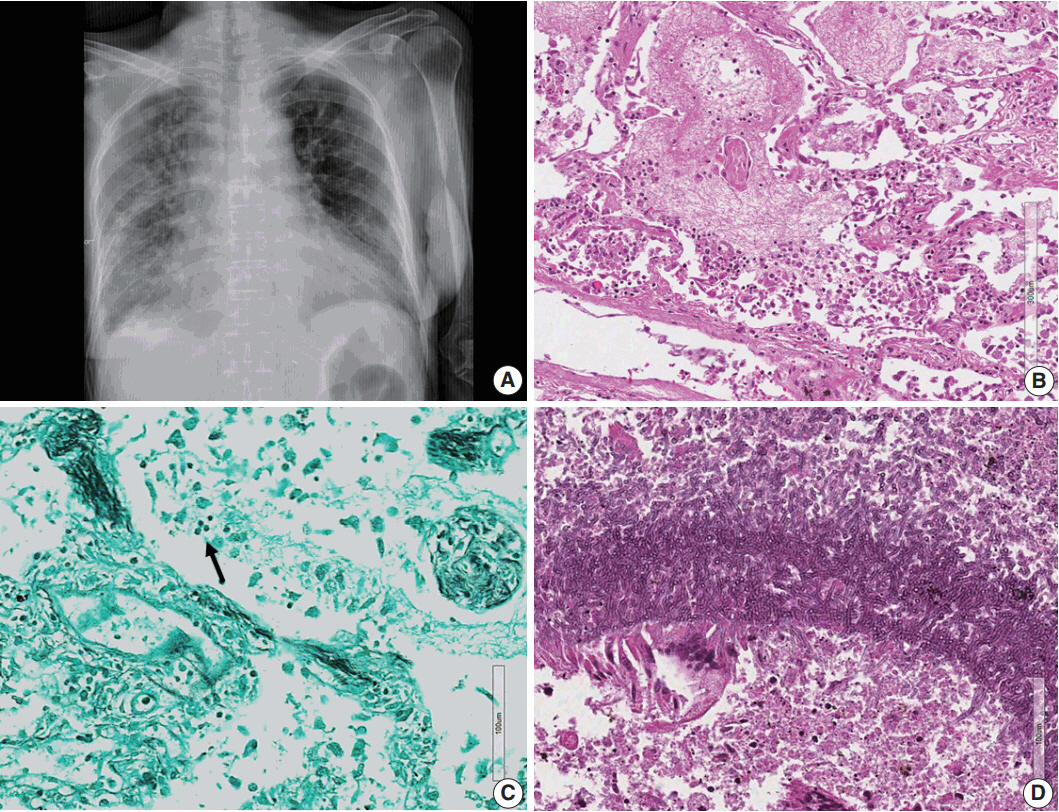
- Lymphomas arising in the central nervous system (CNS) of immunocompromised hosts are most commonly non-Hodgkin's lymphomas and are highly associated with Epstein-Barr virus (EBV). Here we report an autopsy case of EBV-associated CNS diffuse large B-cell lymphoma (DLBCL) in a host suffering from systemic lupus erythematosus who underwent immunosuppressive therapy. After autopsy, EBV-associated CNS DLBCL as well as pulmonary mixed aspergillosis and Pneumocystis jirovecii pneumonia were added to the cause of clinical manifestations of complicated pneumonia and cerebral hemorrhage in this immunocompromised patient. In conclusion, complex disease processes were revealed by autopsy in this case, indicating that the clinicopathological correlations observed through autopsy can improve our understanding of disease progression and contribute to the management of similar patients in the future.
MeSH Terms
Figure
Reference
-
1. Sugita Y, Muta H, Ohshima K, et al. Primary central nervous system lymphomas and related diseases: pathological characteristics and discussion of the differential diagnosis. Neuropathology. 2016; 36:313–24.
Article2. Jamal SE, Li S, Bajaj R, et al. Primary central nervous system Epstein-Barr virus-positive diffuse large B-cell lymphoma of the elderly: a clinicopathologic study of five cases. Brain Tumor Pathol. 2014; 31:265–73.
Article3. Sabattini E, Bacci F, Sagramoso C, Pileri SA. WHO classification of tumours of haematopoietic and lymphoid tissues in 2008: an overview. Pathologica. 2010; 102:83–7.4. Doucet S, Kumthekar P, Raizer J. Primary central nervous system lymphoma. Curr Treat Options Oncol. 2013; 14:185–97.
Article5. Bayraktar S, Bayraktar UD, Ramos JC, Stefanovic A, Lossos IS. Primary CNS lymphoma in HIV positive and negative patients: comparison of clinical characteristics, outcome and prognostic factors. J Neurooncol. 2011; 101:257–65.
Article6. Bibas M, Antinori A. EBV and HIV-related lymphoma. Mediterr J Hematol Infect Dis. 2009; 1:e2009032.
Article7. Tun HW, Personett D, Baskerville KA, et al. Pathway analysis of primary central nervous system lymphoma. Blood. 2008; 111:3200–10.
Article8. Roschewski M, Wilson WH. EBV-associated lymphomas in adults. Best Pract Res Clin Haematol. 2012; 25:75–89.
Article9. Romero M, González-Fontal GR, Saavedra C, et al. Primary CNS plasmablastic lymphoma in an HIV/EBV negative patient: a case report. Diagn Cytopathol. 2016; 44:61–5.
Article10. Campo E, Swerdlow SH, Harris NL, Pileri S, Stein H, Jaffe ES. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood. 2011; 117:5019–32.
Article11. Fraser E, Gruenberg K, Rubenstein JL. New approaches in primary central nervous system lymphoma. Chin Clin Oncol. 2015; 4:11.12. Phillips EH, Fox CP, Cwynarski K. Primary CNS lymphoma. Curr Hematol Malig Rep. 2014; 9:243–53.
Article13. Mahmoud HM, El-Sakhawy YN. Significance of Bcl-2 and Bcl-6 immunostaining in B-Non Hodgkin's lymphoma. Hematol Rep. 2011; 3:e26.
Article14. Hunt KE, Reichard KK. Diffuse large B-cell lymphoma. Arch Pathol Lab Med. 2008; 132:118–24.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Epstein-Barr Virus-Positive Diffuse Large B-Cell Lymphoma Occurring in Thyroid Gland
- A Case of Epstein-Barr Virus-positive Diffuse, Large B-cell Lymphoma after Angioimmunoblastic T-cell Lymphoma
- Epstein-Barr Virus Positive Diffuse Large B-Cell Lymphoma with Epidermotropism
- Diffuse Large B-Cell Lymphoma Associated with Chronic Inflammation Manifested as a Soft Tissue Mass: Incidental Discovery on Histological Examination
- Application of Epstein-Barr Virus Cell Lines (CCL85 EB-3) in Performing the EBER mRNA In Situ Hybridization as a Positive Control