J Vet Sci.
2018 Jan;19(1):51-57. 10.4142/jvs.2018.19.1.51.
Tannic acid-mediated immune activation attenuates Brucella abortus infection in mice
- Affiliations
-
- 1Institute of Animal Medicine, College of Veterinary Medicine, Gyeongsang National University, Jinju 52828, Korea. kimsuk@gnu.ac.kr
- 2Department of Veterinary Paraclinical Sciences, College of Veterinary Medicine, University of the Philippines Los Baños, Laguna 4031, Philippines.
- 3Institute of Agriculture and Life Science, Gyeongsang National University, Jinju 52828, Korea.
- KMID: 2402695
- DOI: http://doi.org/10.4142/jvs.2018.19.1.51
Abstract
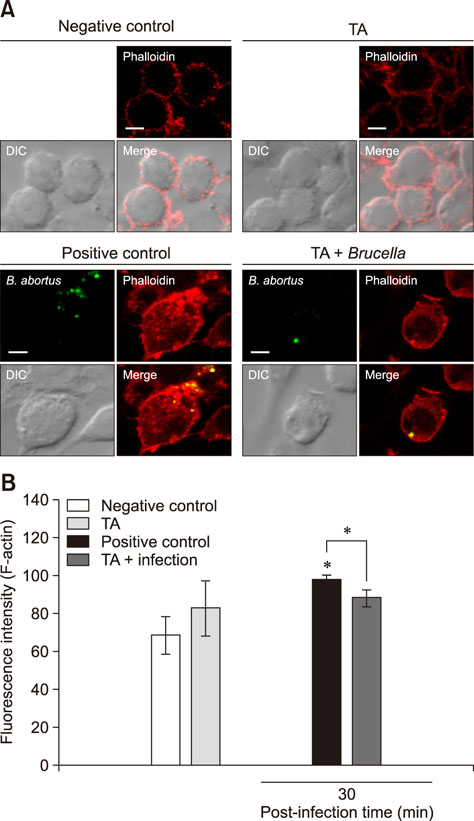
- Brucellosis is an emerging infectious disease affecting humans and animals. In this study, we investigated the in vitro and in vivo effects of tannic acid (TA) against Brucella abortus infection. After infection, F-actin polymerization and mitogen-activated protein kinases (MAPKs) (ERK 1/2 and p38α) phosphorylation were reduced in TA-treated cells compared with that in control cells. The mice were infected via an intraperitoneal route and were orally given TA or phosphate-buffered saline for 14 days. Spleen weights of the TA-treated and control mice were not different; however, splenic proliferation of B. abortus was significantly reduced in the TA-treated group. Immune response analysis showed that, compared with the control group, non-infected TA-treated mice displayed increased levels of interferon-γ (IFN-γ), monocyte chemoattractant protein-1 (MCP-1), and interleukin-10 at 3 days post-infection and a further increase in IFN-γ and MCP-1 at 14 days post-infection. In contrast, compared with the control group, infected TA-treated mice displayed elevated levels of IFN-γ at 3 days post-infection, which continued to increase at 14 days post-infection, as was also observed for tumor necrosis factor. Taken together, the results showing TA activation of cytokine production and inhibition of bacterial proliferation in the host highlight a potential use of TA treatment in the control of Brucella infection.
MeSH Terms
-
Actins
Animals
Brucella abortus*
Brucella*
Brucellosis
Chemokine CCL2
Communicable Diseases, Emerging
Cytokines
Humans
In Vitro Techniques
Interleukin-10
Mice*
Mitogen-Activated Protein Kinases
Phosphorylation
Polymerization
Polymers
Spleen
Tannins
Tumor Necrosis Factor-alpha
Weights and Measures
Actins
Chemokine CCL2
Cytokines
Interleukin-10
Mitogen-Activated Protein Kinases
Polymers
Tannins
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Ahmed W, Zheng K, Liu ZF. Establishment of chronic infection: Brucella's stealth strategy. Front Cell Infect Microbiol. 2016; 6:30.2. Akiyama H, Fujii K, Yamasaki O, Oono T, Iwatsuki K. Antibacterial action of several tannins against Staphylococcus aureus. J Antimicrob Chemother. 2001; 48:487–491.3. Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular Biology of the Cell. 4th ed. New York: Garland Science;2002.4. Athanassakis I, Iconomidou B. Cytokine production in the serum and spleen of mice from day 6 to 14 of gestation: cytokines/placenta/spleen/serum. Dev Immunol. 1996; 4:247–255.
Article5. de Figueiredo P, Ficht TA, Rice-Ficht A, Rossetti CA, Adams LG. Pathogenesis and immunobiology of brucellosis: review of Brucella-host interactions. Am J Pathol. 2015; 185:1505–1517.6. Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009; 29:313–326.
Article7. Gwida M, El-Ashker M, Melzer F, El-Diasty M, El-Beskawy M, Neubauer H. Use of serology and real time PCR to control an outbreak of bovine brucellosis at a dairy cattle farm in the Nile Delta region, Egypt. Ir Vet J. 2016; 69:3.
Article8. Ishizaki H, Yamada S, Yanagiguchi K, Koyama Z, Ikeda T, Hayashi Y. Pre-treatment with tannic acid inhibits the intracellular IL-8 production by chitosan in a human oral epithelial cancer cell line. Oral Med Pathol. 2008; 13:135–141.
Article9. Kim TJ, Silva JL, Kim MK, Jung YS. Enhanced antioxidant capacity and antimicrobial activity of tannic acid by thermal processing. Food Chem. 2010; 118:740–746.
Article10. Ko J, Splitter GA. Molecular host-pathogen interaction in brucellosis: current understanding and future approaches to vaccine development for mice and humans. Clin Microbiol Rev. 2003; 16:65–78.
Article11. Lee JJ, Bae JH, Kim DH, Lim JJ, Kim DG, Lee HJ, Min W, Rhee MH, Chang HH, Park H, Kim S. Intracellular replication inhibitory effects of Galla Rhois ethanol extract for Brucella abortus infection. J Ethnopharmacol. 2011; 138:602–609.
Article12. Lee JJ, Kim DH, Kim DG, Lee HJ, Min W, Rhee MH, Cho JY, Watarai M, Kim S. Toll-like receptor 4-linked Janus kinase 2 signaling contributes to internalization of Brucella abortus by macrophages. Infect Immun. 2013; 81:2448–2458.
Article13. Lee JJ, Kim DH, Park SB, Lim JJ, Kim DG, Min WG, Lee HJ, Kim DK, Chang HH, Kim S. Redundant effects of ketamine on the pathogenesis and severity of Brucella abortus infection. Comp Immunol Microbiol Infect Dis. 2013; 36:71–81.
Article14. Lim JJ, Kim DH, Lee JJ, Kim DG, Min W, Lee HJ, Rhee MH, Kim S. Protective effects of recombinant Brucella abortus Omp28 against infection with a virulent strain of Brucella abortus 544 in mice. J Vet Sci. 2012; 13:287–292.
Article15. Macedo GC, Magnani DM, Carvalho NB, Bruna-Romero O, Gazzinelli RT, Oliveira SC. Central role of MyD88-dependent dendritic cell maturation and proinflammatory cytokine production to control Brucella abortus infection. J Immunol. 2008; 180:1080–1087.
Article16. Moreno E. Retrospective and prospective perspectives on zoonotic brucellosis. Front Microbiol. 2014; 5:213.
Article17. Motamedi H, Darabpour E, Gholipour M, Seyyed Nejad SM. In vitro assay for the anti-Brucella activity of medicinal plants against tetracycline-resistant Brucella melitensis. J Zhejiang Univ Sci B. 2010; 11:506–511.
Article18. Myint KB, Sing LC, Wei Z. Tannic acid as phytochemical potentiator for antibiotic resistance adaptation. APCBEE Procedia. 2013; 7:175–181.
Article19. Rambow-Larsen AA, Petersen EM, Gourley CR, Splitter GA. Brucella regulators: self-control in a hostile environment. Trends Microbiol. 2009; 17:371–377.20. Reyes AW, Arayan LT, Simborio HL, Hop HT, Min W, Lee HJ, Kim DH, Chang HH, Kim S. Dextran sulfate sodium upregulates MAPK signaling for the uptake and subsequent intracellular survival of Brucella abortus in murine macrophages. Microb Pathog. 2016; 91:68–73.
Article21. Reyes AW, Kim DG, Simborio HL, Hop HT, Arayan LT, Min W, Lee JJ, Chang HH, Kim S. Methyl gallate limits infection in mice challenged with Brucella abortus while enhancing the inflammatory response. J Appl Microbiol. 2016; 120:552–559.
Article22. Reyes AW, Simborio HL, Hop HT, Arayan LT, Kim S. Molecular cloning, purification and immunogenicity of recombinant Brucella abortus 544 malate dehydrogenase protein. J Vet Sci. 2016; 17:119–122.
Article23. Russo G, Pasquali P, Nenova R, Alexandrov T, Ralchev S, Vullo V, Rezza G, Kantardjiev T. Reemergence of human and animal brucellosis, Bulgaria. Emerg Infect Dis. 2009; 15:314–316.
Article24. Scalbert A. Antimicrobial properties of tannins. Phytochemistry. 1991; 30:3875–3883.
Article25. Xavier MN, Winter MG, Spees AM, Nguyen K, Atluri VL, Silva TM, Bäumler AJ, Müller W, Santos RL, Tsolis RM. CD4+ T cell-derived IL-10 promotes Brucella abortus persistence via modulation of macrophage function. PLoS Pathog. 2013; 9:e1003454.26. Yoon H, Moon OK, Lee SH, Lee WC, Her M, Jeong W, Jung SC, Kim DS. Epidemiology of brucellosis among cattle in Korea from 2001 to 2011. J Vet Sci. 2014; 15:537–543.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Protective effects of recombinant Brucella abortus Omp28 against infection with a virulent strain of Brucella abortus 544 in mice
- Diversity of Humoral Immune Responses to Recombinant Proteins of Brucella abortus Among Residents in Cheju Province
- Verification of immunosuppression in chicks caused by Cryptosporidium baileyi infection using Brucella abortus strain 1119-3
- Different invasion efficiencies of Brucella abortus wild-type and mutantsin RAW 264.7 and THP-1 phagocytic cells and HeLa non-phagocytic cells
- Successful Medical Treatment of Prosthetic Mitral Valve Endocarditis Caused by Brucella abortus