J Vet Sci.
2018 Jan;19(1):21-26. 10.4142/jvs.2018.19.1.21.
Coactosin-like protein 1 inhibits neuronal migration during mouse corticogenesis
- Affiliations
-
- 1College of Veterinary Medicine, Northwest A&F University, Yangling 712100, China. shantingzhao@hotmail.com, csl_1359@126.com
- KMID: 2402691
- DOI: http://doi.org/10.4142/jvs.2018.19.1.21
Abstract
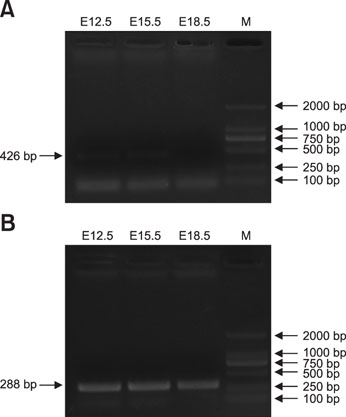
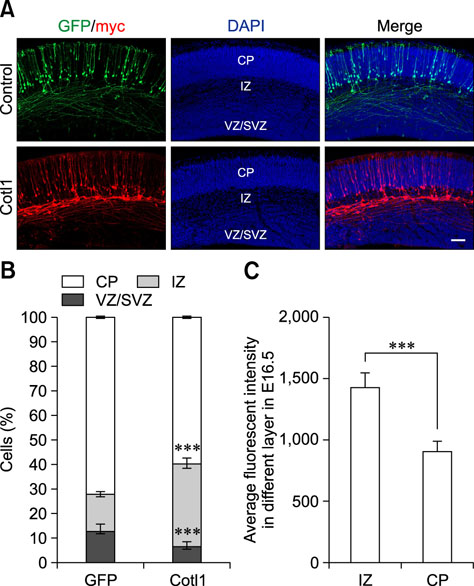
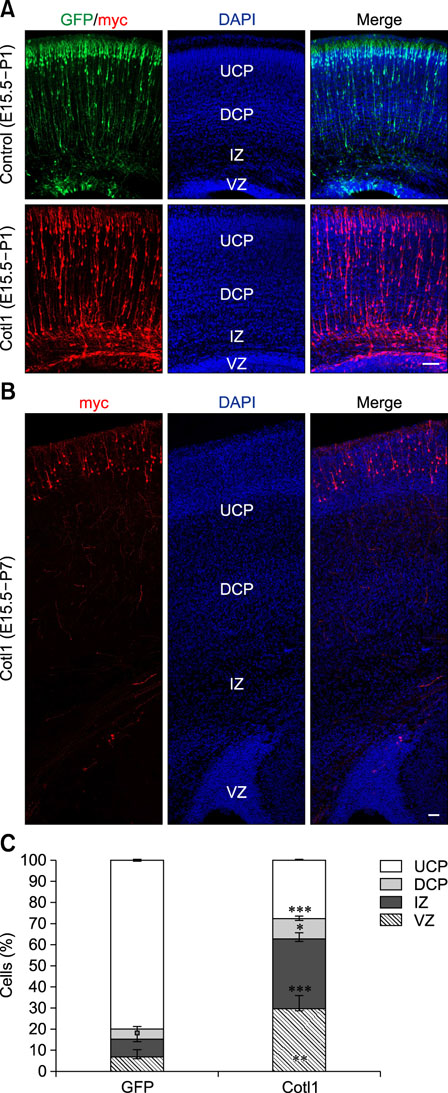
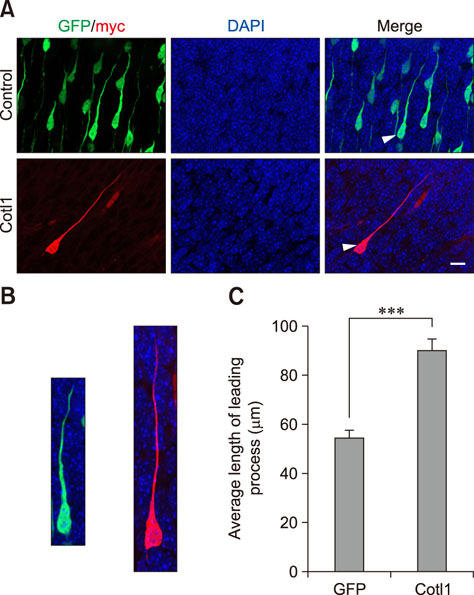
- Coactosin-like protein 1 (Cotl1), a member of the actin-depolymerizing factor (ADF)/cofilin family, was first purified from a soluble fraction of Dictyostelium discoideum cells. Neuronal migration requires cytoskeletal remodeling and actin regulation. Although Cotl1 strongly binds to F-actin, the role of Cotl1 in neuronal migration remains undescribed. In this study, we revealed that Cotl1 overexpression impaired migration of both early- and late-born neurons during mouse corticogenesis. Moreover, Cotl1 overexpression delayed, rather than blocked, neuronal migration in late-born neurons. Cotl1 expression disturbed the morphology of migrating neurons, lengthening the leading processes. This study is the first to investigate the function of Cotl1, and the results indicate that Cotl1 is involved in the regulation of neuronal migration and morphogenesis.
Figure
Reference
-
1. Angevine JB Jr, Sidman RL. Autoradiographic study of cell migration during histogenesis of cerebral cortex in the mouse. Nature. 1961; 192:766–768.
Article2. Austin CP, Cepko CL. Cellular migration patterns in the developing mouse cerebral cortex. Development. 1990; 110:713–732.
Article3. Ayala R, Shu T, Tsai LH. Trekking across the brain: the journey of neuronal migration. Cell. 2007; 128:29–43.
Article4. Chai X, Zhao S, Fan L, Zhang W, Lu X, Shao H, Wang S, Song L, Failla AV, Zobiak B, Mannherz HG, Frotscher M. Reelin and cofilin cooperate during the migration of cortical neurons: a quantitative morphological analysis. Development. 2016; 143:1029–1040.
Article5. de Carlos JA, López-Mascaraque L, Valverde F. Dynamics of cell migration from the lateral ganglionic eminence in the rat. J Neurosci. 1996; 16:6146–6156.
Article6. de Hostos EL, Bradtke B, Lottspeich F, Gerisch G. Coactosin, a 17 kDa F-actin binding protein from Dictyostelium discoideum. Cell Motil Cytoskeleton. 1993; 26:181–191.7. Franco SJ, Martinez-Garay I, Gil-Sanz C, Harkins-Perry SR, Müller U. Reelin regulates cadherin function via Dab1/Rap1 to control neuronal migration and lamination in the neocortex. Neuron. 2011; 69:482–497.
Article8. Goroncy AK, Koshiba S, Tochio N, Tomizawa T, Sato M, Inoue M, Watanabe S, Hayashizaki Y, Tanaka A, Kigawa T, Yokoyama S. NMR solution structures of actin depolymerizing factor homology domains. Protein Sci. 2009; 18:2384–2392.
Article9. Hatanaka Y, Hisanaga S, Heizmann CW, Murakami F. Distinct migratory behavior of early- and late-born neurons derived from the cortical ventricular zone. J Comp Neurol. 2004; 479:1–14.
Article10. He M, Zhang ZH, Guan CB, Xia D, Yuan XB. Leading tip drives soma translocation via forward F-actin flow during neuronal migration. J Neurosci. 2010; 30:10885–10898.
Article11. Jovceva E, Larsen MR, Waterfield MD, Baum B, Timms JF. Dynamic cofilin phosphorylation in the control of lamellipodial actin homeostasis. J Cell Sci. 2007; 120:1888–1897.
Article12. Kawauchi T, Hoshino M. Molecular pathways regulating cytoskeletal organization and morphological changes in migrating neurons. Dev Neurosci. 2008; 30:36–46.
Article13. Kim J, Shapiro MJ, Bamidele AO, Gurel P, Thapa P, Higgs HN, Hedin KE, Shapiro VS, Billadeau DD. Coactosin-like 1 antagonizes cofilin to promote lamellipodial protrusion at the immune synapse. PLoS One. 2014; 9:e85090.
Article14. Lappalainen P, Kessels MM, Cope MJ, Drubin DG. The ADF homology (ADF-H) domain: a highly exploited actin-binding module. Mol Biol Cell. 1998; 9:1951–1959.
Article15. Marín O, Rubenstein JL. Cell migration in the forebrain. Annu Rev Neurosci. 2003; 26:441–483.16. Miyata T, Kawaguchi A, Okano H, Ogawa M. Asymmetric inheritance of radial glial fibers by cortical neurons. Neuron. 2001; 31:727–741.
Article17. Nadarajah B, Brunstrom JE, Grutzendler J, Wong RO, Pearlman AL. Two modes of radial migration in early development of the cerebral cortex. Nat Neurosci. 2001; 4:143–150.
Article18. Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003; 112:453–465.
Article19. Provost P, Doucet J, Stock A, Gerisch G, Samuelsson B, Rådmark O. Coactosin-like protein, a human F-actin-binding protein: critical role of lysine-75. Biochem J. 2001; 359:255–263.
Article20. Rakic P. A century of progress in corticoneurogenesis: from silver impregnation to genetic engineering. Cereb Cortex. 2006; 16:Suppl 1. i3–i17.
Article21. Rakic P. Developmental and evolutionary adaptations of cortical radial glia. Cereb Cortex. 2003; 13:541–549.
Article22. Rakic P. Guidance of neurons migrating to the fetal monkey neocortex. Brain Res. 1971; 33:471–476.
Article23. Rakic P. Mode of cell migration to the superficial layers of fetal monkey neocortex. J Comp Neurol. 1972; 145:61–83.
Article24. Sekine K, Kawauchi T, Kubo K, Honda T, Herz J, Hattori M, Kinashi T, Nakajima K. Reelin controls neuronal positioning by promoting cell-matrix adhesion via inside-out activation of integrin α5β1. Neuron. 2012; 76:353–369.
Article25. Valiente M, Marín O. Neuronal migration mechanisms in development and disease. Curr Opin Neurobiol. 2010; 20:68–78.
Article26. Walsh C, Cepko CL. Clonally related cortical cells show several migration patterns. Science. 1988; 241:1342–1345.
Article27. Xie J, Li X, Zhang W, Chai X, Huang Y, Li K, Cheng X, Zhao S. Aberrant expression of LIMK1 impairs neuronal migration during neocortex development. Histochem Cell Biol. 2017; 147:471–479.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Neuronal Heterotopia
- Expression of Laminin Chains in the Neuronal Cells of Mouse Brain
- Effect of glial-neuronal cell co-culture on GFAP expression of astrocytes
- Dopaminergic neuronal development in the embryonic mesencephalon of mouse
- Neurogenesis and neuronal migration of dopaminergic neurons during mesencephalon development in mice