J Vet Sci.
2018 Jan;19(1):3-12. 10.4142/jvs.2018.19.1.3.
Chicken RNA-binding protein T-cell internal antigen-1 contributes to stress granule formation in chicken cells and tissues
- Affiliations
-
- 1Department of Avian Infectious Diseases, Shanghai Veterinary Research Institute, Chinese Academy of Agricultural Science, Shanghai 200241, China. shoveldeen@shvri.ac.cn
- 2College of Animal Science and Technology, Shandong Agricultural University, Taian 271018, China.
- 3College of Animal Science and Technology, Jilin Agricultural University, Changchun 130118, China.
- 4Jiangsu Co-innovation Center for Prevention and Control of Important Animal Infectious Diseases and Zoonoses, Yangzhou 225009, China.
- KMID: 2402689
- DOI: http://doi.org/10.4142/jvs.2018.19.1.3
Abstract
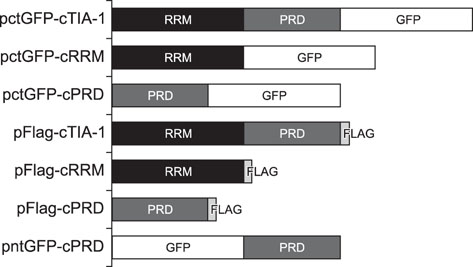
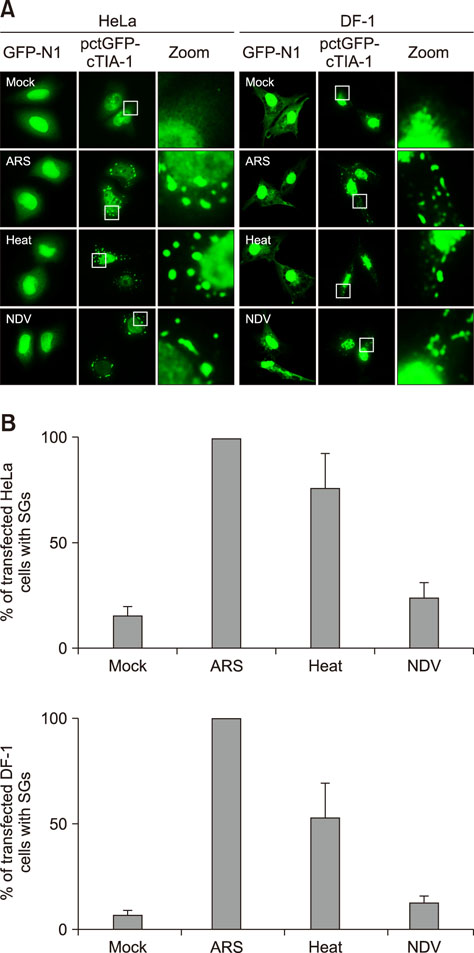
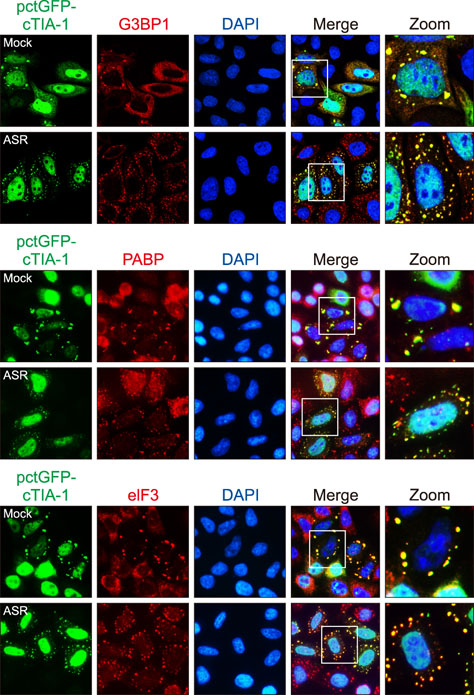
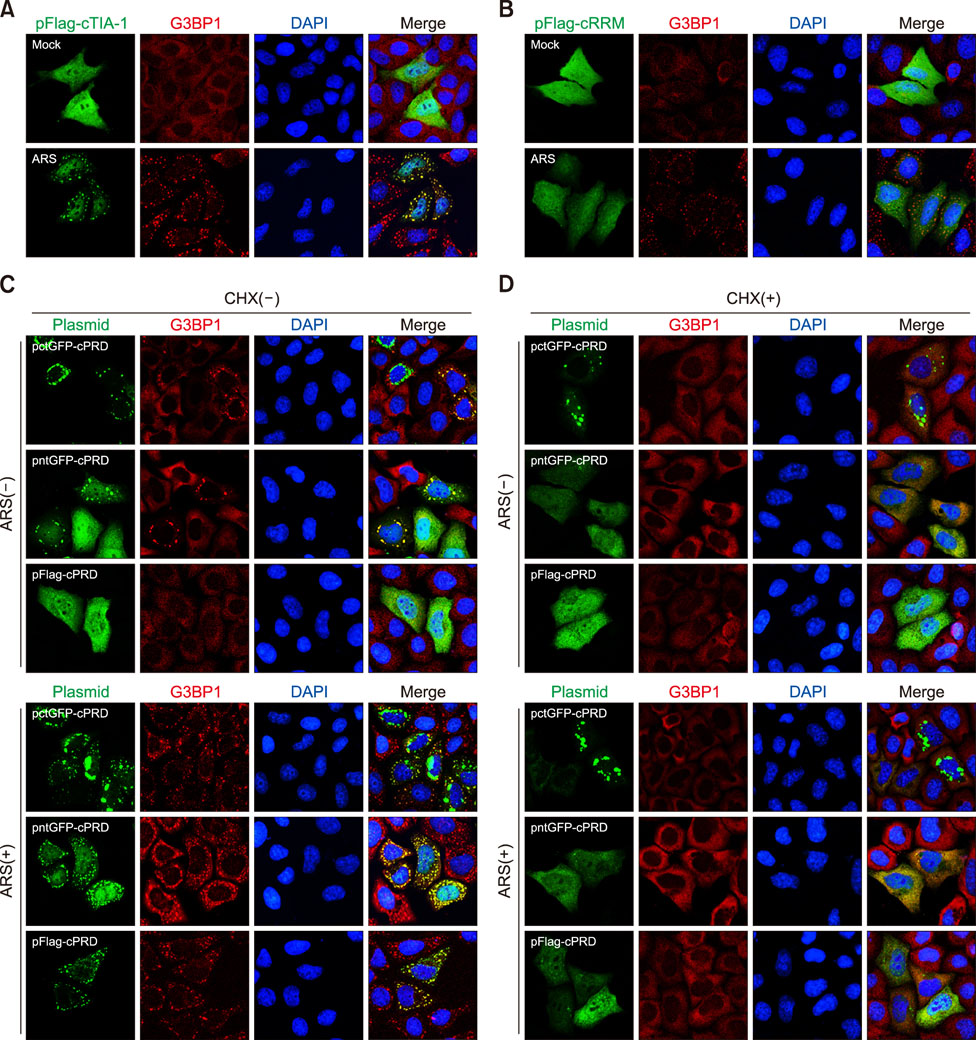
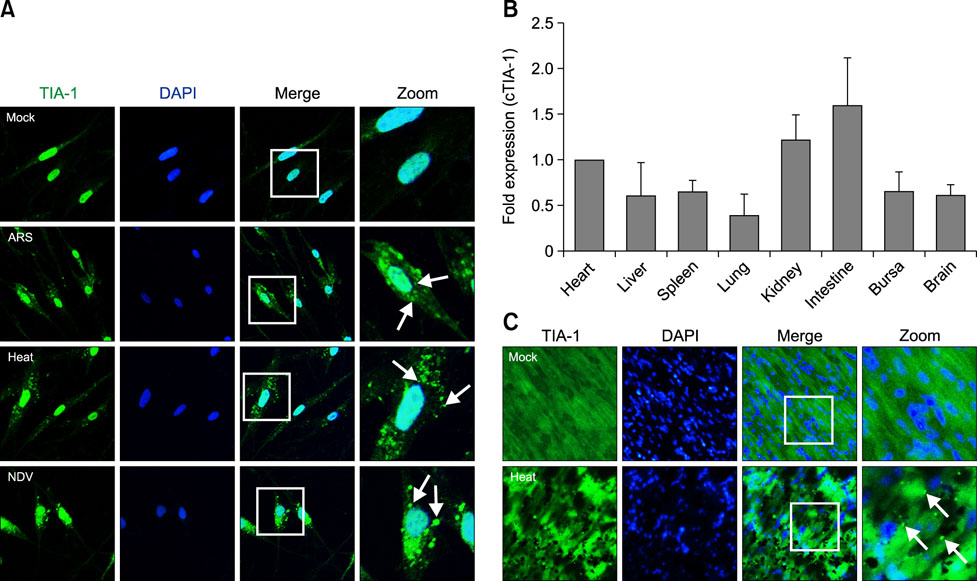
- T-cell internal antigen-1 (TIA-1) has roles in regulating alternative pre-mRNA splicing, mRNA translation, and stress granule (SG) formation in human cells. As an evolutionarily conserved response to environmental stress, SGs have been reported in various species. However, SG formation in chicken cells and the role of chicken TIA-1 (cTIA-1) in SG assembly has not been elucidated. In the present study, we cloned cTIA-1 and showed that it facilitates the assembly of canonical SGs in both human and chicken cells. Overexpression of the chicken prion-related domain (cPRD) of cTIA-1 that bore an N-terminal green fluorescent protein (GFP) tag (pntGFP-cPRD) or Flag tag (pFlag-cPRD) induced the production of typical SGs. However, C-terminal GFP-tagged cPRD induced notably large cytoplasmic granules that were devoid of endogenous G3BP1 and remained stable when exposed to cycloheximide, indicating that these were not typical SGs, and that the pntGFP tag influences cPRD localization. Finally, endogenous cTIA-1 was recruited to SGs in chicken cells and tissues under environmental stress. Taken together, our study provide evidence that cTIA-1 has a role in canonical SG formation in chicken cells and tissues. Our results also indicate that cPRD is necessary for SG aggregation.
MeSH Terms
Figure
Reference
-
1. Anderson P, Kedersha N. RNA granules. J Cell Biol. 2006; 172:803–808.
Article2. Buchan JR, Muhlrad D, Parker R. P bodies promote stress granule assembly in Saccharomyces cerevisiae. J Cell Biol. 2008; 183:441–455.3. Collier NC, Schlesinger MJ. The dynamic state of heat shock proteins in chicken embryo fibroblasts. J Cell Biol. 1986; 103:1495–1507.
Article4. Cubitt AB, Heim R, Adams SR, Boyd AE, Gross LA, Tsien RY. Understanding, improving and using green fluorescent proteins. Trends Biochem Sci. 1995; 20:448–455.
Article5. De Maio A. Heat shock proteins: facts, thoughts, and dreams. Shock. 1999; 11:1–12.
Article6. Efthymiou CA, Mocanu MM, de Belleroche J, Wells DJ, Latchmann DS, Yellon DM. Heat shock protein 27 protects the heart against myocardial infarction. Basic Res Cardiol. 2004; 99:392–394.
Article7. Förch P, Puig O, Kedersha N, Martínez C, Granneman S, Séraphin B, Anderson P, Valcárcel J. The apoptosis-promoting factor TIA-1 is a regulator of alternative pre-mRNA splicing. Mol Cell. 2000; 6:1089–1098.
Article8. Gallouzi IE, Brennan CM, Stenberg MG, Swanson MS, Eversole A, Maizels N, Steitz JA. HuR binding to cytoplasmic mRNA is perturbed by heat shock. Proc Natl Acad Sci U S A. 2000; 97:3073–3078.
Article9. Gerdes HH, Kaether C. Green fluorescent protein: applications in cell biology. FEBS Lett. 1996; 389:44–47.
Article10. Gilks N, Kedersha N, Ayodele M, Shen L, Stoecklin G, Dember LM, Anderson P. Stress granule assembly is mediated by prion-like aggregation of TIA-1. Mol Biol Cell. 2004; 15:5383–5398.
Article11. Kandasamy K, Joseph K, Subramaniam K, Raymond JR, Tholanikunnel BG. Translational control of beta2-adrenergic receptor mRNA by T-cell-restricted intracellular antigen-related protein. J Biol Chem. 2005; 280:1931–1943.
Article12. Kedersha N, Anderson P. Stress granules: sites of mRNA triage that regulate mRNA stability and translatability. Biochem Soc Trans. 2002; 30:963–969.
Article13. Kedersha N, Chen S, Gilks N, Li W, Miller IJ, Stahl J, Anderson P. Evidence that ternary complex (eIF2-GTP-tRNAiMet)-deficient preinitiation complexes are core constituents of mammalian stress granules. Mol Biol Cell. 2002; 13:195–210.
Article14. Kedersha N, Cho MR, Li W, Yacono PW, Chen S, Gilks N, Golan DE, Anderson P. Dynamic shuttling of TIA-1 accompanies the recruitment of mRNA to mammalian stress granules. J Cell Biol. 2000; 151:1257–1268.
Article15. Kedersha NL, Gupta M, Li W, Miller I, Anderson P. RNA-binding proteins TIA-1 and TIAR link the phosphorylation of eIF-2 alpha to the assembly of mammalian stress granules. J Cell Biol. 1999; 147:1431–1442.
Article16. Le Guiner C, Gesnel MC, Breathnach R. TIA-1 or TIAR is required for DT40 cell viability. J Biol Chem. 2003; 278:10465–10476.
Article17. Mangiardi DA, McLaughlin-Williamson K, May KE, Messana EP, Mountain DC, Cotanche DA. Progression of hair cell ejection and molecular markers of apoptosis in the avian cochlea following gentamicin treatment. J Comp Neurol. 2004; 475:1–18.
Article18. Nover L, Scharf KD, Neumann D. Formation of cytoplasmic heat shock granules in tomato cell cultures and leaves. Mol Cell Biol. 1983; 3:1648–1655.
Article19. Ooie T, Takahashi N, Saikawa T, Nawata T, Arikawa M, Yamanaka K, Hara M, Shimada T, Sakata T. Single oral dose of geranylgeranylacetone induces heat-shock protein 72 and renders protection against ischemia/reperfusion injury in rat heart. Circulation. 2001; 104:1837–1843.
Article20. Piecyk M, Wax S, Beck AR, Kedersha N, Gupta M, Maritim B, Chen S, Gueydan C, Kruys V, Streuli M, Anderson P. TIA-1 is a translational silencer that selectively regulates the expression of TNF-alpha. EMBO J. 2000; 19:4154–4163.
Article21. Piotrowska J, Hansen SJ, Park N, Jamka K, Sarnow P, Gustin KE. Stable formation of compositionally unique stress granules in virus-infected cells. J Virol. 2010; 84:3654–3665.
Article22. Serio TR, Lindquist SL. [PSI+], SUP35, and chaperones. Adv Protein Chem. 2001; 57:335–366.
Article23. Sun Y, Ding N, Ding SS, Yu S, Meng C, Chen H, Qiu X, Zhang S, Yu Y, Zhan Y, Ding C. Goose RIG-I functions in innate immunity against Newcastle disease virus infections. Mol Immunol. 2013; 53:321–327.
Article24. Sun Y, Yu S, Ding N, Meng C, Meng S, Zhang S, Zhan Y, Qiu X, Tan L, Chen H, Song C, Ding C. Autophagy benefits the replication of Newcastle disease virus in chicken cells and tissues. J Virol. 2014; 88:525–537.
Article25. Tourrière H, Chebli K, Zekri L, Courselaud B, Blanchard JM, Bertrand E, Tazi J. The RasGAP-associated endoribonuclease G3BP assembles stress granules. J Cell Biol. 2003; 160:823–831.
Article26. Vaheri A, Ruoslahti E, Sarvas M, Nurminen M. Mitogenic effect by lipopolysaccharide and pokeweed lectin on density-inhibited chick embryo fibroblasts. J Exp Med. 1973; 138:1356–1364.
Article27. Zhang T, Delestienne N, Huez G, Kruys V, Gueydan C. Identification of the sequence determinants mediating the nucleo-cytoplasmic shuttling of TIAR and TIA-1 RNA-binding proteins. J Cell Sci. 2005; 118:5453–5463.
Article28. Zhu H, Hasman RA, Young KM, Kedersha NL, Lou H. U1 snRNP-dependent function of TIAR in the regulation of alternative RNA processing of the human calcitonin/CGRP pre-mRNA. Mol Cell Biol. 2003; 23:5959–5971.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Neuronal RNA granule contains ApCPEB1, a novel cytoplasmic polyadenylation element binding protein, in Aplysia sensory neuron
- Downregulation of cellular prion protein inhibited the proliferation and invasion and induced apoptosis of Marek's disease virus-transformed avian T cells
- Isolation and Characterization of Chicken NPAS3
- (3H)Ryanodine binding sites of SR vesicles of the chicken pectoral muscle
- Isolation and identification of Cryptosporidium from various animals in Korea. III. Identification of Cryptosporidium baileyi from Korean chicken