Intest Res.
2018 Jan;16(1):142-146. 10.5217/ir.2018.16.1.142.
Fecal microbiota transplantation for recurrent Clostridium difficile infection in a patient with ulcerative colitis
- Affiliations
-
- 1Division of Gastroenterology and Hepatology, Department of Internal Medicine, Keio University School of Medicine, Tokyo, Japan. takagast@keio.jp
- 2Department of Gastroenterology and Hepatology, Tokyo Medical and Dental University, Tokyo, Japan.
- 3Department of Bacteriology II, National Institute of Infectious Diseases, Tokyo, Japan.
- 4Graduate School of Frontier Sciences, University of Tokyo, Chiba, Japan.
- 5Faculty of Science and Engineering, Waseda University, Tokyo, Japan.
- KMID: 2402658
- DOI: http://doi.org/10.5217/ir.2018.16.1.142
Abstract
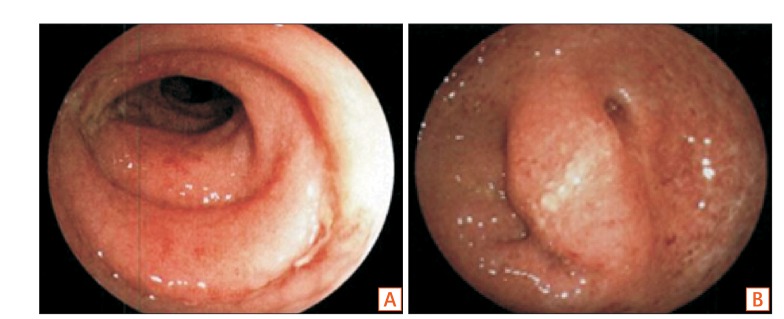
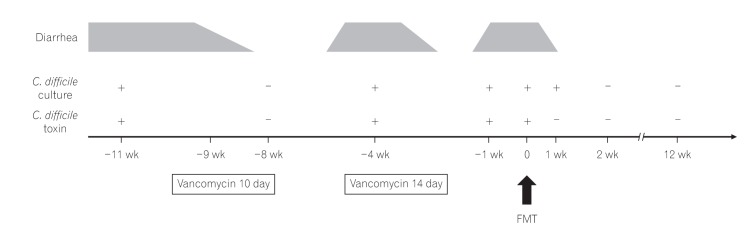
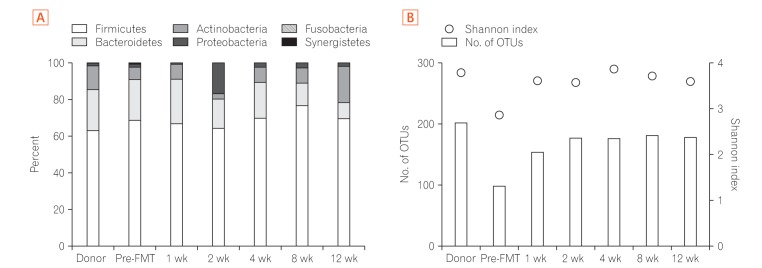
- Fecal microbiota transplantation (FMT) has been reported as a safe and effective therapy in patients with refractory and recurrent Clostridium difficile infection (CDI). FMT has also been reported as a promising therapy in patients with ulcerative colitis (UC). Both, CDI and UC, are believed to be caused by dysbiosis, such as altered compositions or decreased diversity of the intestinal microbiota. This report describes a patient with UC in remission with a second recurrent episode of CDI, who was treated with FMT. A single FMT performed via colonoscopy completely resolved the patient's diarrhea and eradicated C. difficile bacteriologically without any severe complications. Molecular biological analysis of the patient's fecal microbiota showed that FMT could dramatically change the altered composition of intestinal microbiota and restore its diversity. Despite the restoration of the intestinal microbiota, FMT could not prevent a relapse of UC in this patient. However, it improved the intestinal symptoms of CDI and could prevent further recurrences of CDI.
MeSH Terms
Figure
Cited by 1 articles
-
Management of
Clostridioides difficile infection in patients with inflammatory bowel disease
Sahil Khanna
Intest Res. 2021;19(3):265-274. doi: 10.5217/ir.2020.00045.
Reference
-
1. Sinh P, Barrett TA, Yun L. Clostridium difficile infection and inflammatory bowel disease: a review. Gastroenterol Res Pract. 2011; 2011:136064. PMID: 21915178.2. van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013; 368:407–415. PMID: 23323867.
Article3. Brandt LJ. American Journal of Gastroenterology Lecture: intestinal microbiota and the role of fecal microbiota transplant (FMT) in treatment of C. difficile infection. Am J Gastroenterol. 2013; 108:177–185. PMID: 23318479.
Article4. Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013; 108:478–498. PMID: 23439232.
Article5. Debast SB, Bauer MP, Kuijper EJ. European Society of Clinical Microbiology and Infectious Diseases. European society of clinical microbiology and infectious diseases: update of the treatment guidance document for Clostridium difficile infection. Clin Microbiol Infect. 2014; 20(Suppl 2):1–26.
Article6. Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015; 372:825–834. PMID: 25714160.
Article7. Tanaka T, Kato H, Fujimoto T. Successful fecal microbiota trans-plantation as an initial therapy for Clostridium difficile infection on an outpatient basis. Intern Med. 2016; 55:999–1000. PMID: 27086820.
Article8. Matsuoka K, Mizuno S, Hayashi A, Hisamatsu T, Naganuma M, Kanai T. Fecal microbiota transplantation for gastrointestinal diseases. Keio J Med. 2014; 63:69–74. PMID: 25500625.
Article9. Brace C, Gloor GB, Ropeleski M, Allen-Vercoe E, Petrof EO. Microbial composition analysis of Clostridium difficile infections in an ulcerative colitis patient treated with multiple fecal microbiota transplantations. J Crohns Colitis. 2014; 8:1133–1137. PMID: 24529606.
Article10. Kato H, Kato N, Watanabe K, et al. Identification of toxin A-negative, toxin B-positive Clostridium difficile by PCR. J Clin Microbiol. 1998; 36:2178–2182. PMID: 9665986.
Article11. Kim SW, Suda W, Kim S, et al. Robustness of gut microbiota of healthy adults in response to probiotic intervention revealed by high-throughput pyrosequencing. DNA Res. 2013; 20:241–253. PMID: 23571675.
Article12. Lawley TD, Clare S, Walker AW, et al. Targeted restoration of the intestinal microbiota with a simple, defined bacteriotherapy resolves relapsing Clostridium difficile disease in mice. PLoS Pathog. 2012; 8:e1002995. DOI: 10.1371/journal.ppat.1002995. PMID: 23133377.
Article13. Mulherin DW, Hutchison AM, Thomas GJ, Hansen RA, Childress DT. Concordance of the SHEA-IDSA severity classification for Clostridium difficile infection and the ATLAS bedside scoring system in hospitalized adult patients. Infection. 2014; 42:999–1005. PMID: 25129565.
Article14. Khanna S, Shin A, Kelly CP. Management of Clostridium difficile infection in inflammatory bowel disease: expert review from the clinical practice updates committee of the AGA institute. Clin Gastroenterol Hepatol. 2017; 15:166–174. PMID: 28093134.
Article15. Issa M, Vijayapal A, Graham MB, et al. Impact of Clostridium difficile on inflammatory bowel disease. Clin Gastroenterol Hepatol. 2007; 5:345–351. PMID: 17368234.
Article16. Kassam Z, Lee CH, Yuan Y, Hunt RH. Fecal microbiota transplantation for Clostridium difficile infection: systematic review and meta-analysis. Am J Gastroenterol. 2013; 108:500–508. PMID: 23511459.
Article17. Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A. 2007; 104:13780–13785. PMID: 17699621.
Article18. Garrett WS, Lord GM, Punit S, et al. Communicable ulcerative colitis induced by T-bet deficiency in the innate immune system. Cell. 2007; 131:33–45. PMID: 17923086.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Refractory Clostridium difficile Infection Cured With Fecal Microbiota Transplantation in Vancomycin-Resistant Enterococcus Colonized Patient
- A Case of Toxic Megacolon Caused by Clostridium difficile Infection and Treated with Fecal Microbiota Transplantation
- Two Cases of Refractory Pseudomembranous Colitis that Healed Following Fecal Microbiota Transplantation
- Fecal Microbiota Transplantation as a Treatment of Recurrent Clostridium difficile Infection: Where Are We Now and Where Are We Heading?
- Why is it so difficult to evaluate faecal microbiota transplantation as a treatment for ulcerative colitis?