Intest Res.
2018 Jan;16(1):109-115. 10.5217/ir.2018.16.1.109.
Rapid and accurate diagnosis of Clostridium difficile infection by real-time polymerase chain reaction
- Affiliations
-
- 1Department of Internal Medicine, Seoul Paik Hospital, Inje University College of Medicine, Seoul, Korea. yousun69@korea.com
- 2Department of Laboratory Medicine, Seoul Paik Hospital, Inje University College of Medicine, Seoul, Korea.
- KMID: 2402654
- DOI: http://doi.org/10.5217/ir.2018.16.1.109
Abstract
- BACKGROUND/AIMS
The incidence and severity of Clostridium difficile infection (CDI) have increased worldwide, resulting in a need for rapid and accurate diagnostic methods.
METHODS
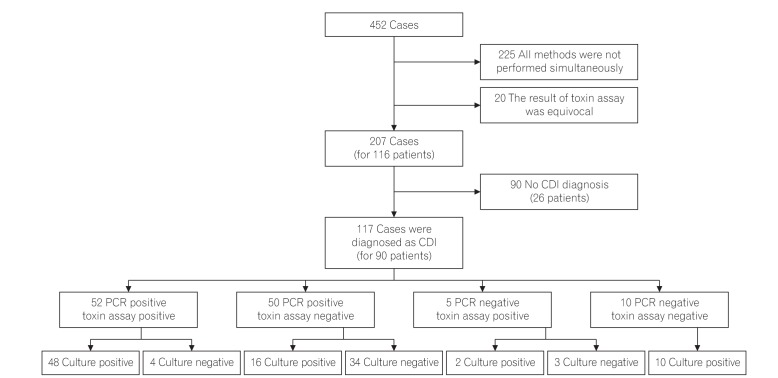
A retrospective study was conducted to compare CDI diagnosis methods between January 2014 and December 2014. The stool samples, which were obtained in presumptive CDI patients, were compared for their diagnostic accuracy and rapidity, including real-time polymerase chain reaction (PCR) of toxin genes, C. difficile toxin assay, and culture for C. difficile.
RESULTS
A total of 207 cases from 116 patients were enrolled in this study and 117 cases (56.5%) were diagnosed as having CDI. Among the 117 cases, the sensitivities of real-time PCR, C. difficile toxin assay, and culture for C. difficile were 87.2% (102 cases; 95% CI, 80.7%-92.8%), 48.7% (57 cases; 95% CI, 41.0%-59.8%), and 65.0% (76 cases; 95% CI, 60.2%-78.5%), respectively (P < 0.005). Notably, 34 cases (29.0%) were diagnosed with CDI by real-time PCR only. The time required to obtain results was 2.27 hours (136.62±82.51 minutes) for real-time PCR, 83.67 hours (5,020.66±3,816.38 minutes) for toxin assay, and 105.79 hours (6,347.68±3,331.46 minutes) for culture (P < 0.005), respectively.
CONCLUSIONS
We confirmed that real-time PCR of toxin genes is the most effective diagnostic method for accurate and early diagnosis of CDI. It also helps to diagnose hypervirulent CDI, such as ribotype 027 infection.
MeSH Terms
Figure
Cited by 2 articles
-
Usefulness of Stool Multiplex Polymerase Chain Reaction Assays in Patients with Acute Diarrhea
Seo Hyun Kim, You Sun Kim, Seung Hyuk Kim, Won Eui Yoon, Hee Jun Myung, Jeong Seop Moon, Dong Hee Whang
Korean J Gastroenterol. 2022;79(3):118-125. doi: 10.4166/kjg.2022.011.Is
Clostridium difficile infection a real threat in patients with ulcerative colitis? A prospective, multicenter study in Korea
Dae Bum Kim, Kang-Moon Lee, Sang Hyoung Park, You Sun Kim, Eun Soo Kim, Jun Lee, Sung-Ae Jung, Geom Seog Seo, Ji Min Lee
Intest Res. 2018;16(2):267-272. doi: 10.5217/ir.2018.16.2.267.
Reference
-
1. O'Connor JR, Johnson S, Gerding DN. Clostridium difficile infection caused by the epidemic BI/NAP1/027 strain. Gastroenterology. 2009; 136:1913–1924. PMID: 19457419.2. Pépin J, Valiquette L, Alary ME, et al. Clostridium difficile-associated diarrhea in a region of Quebec from 1991 to 2003: a changing pattern of disease severity. CMAJ. 2004; 171:466–472. PMID: 15337727.
Article3. Ricciardi R, Rothenberger DA, Madoff RD, Baxter NN. Increasing prevalence and severity of Clostridium difficile colitis in hospitalized patients in the United States. Arch Surg. 2007; 142:624–631. PMID: 17638799.
Article4. Burke KE, Lamont JT. Clostridium difficile infection: a worldwide disease. Gut Liver. 2014; 8:1–6. PMID: 24516694.5. Kim YS, Han DS, Kim YH, et al. Incidence and clinical features of Clostridium difficile infection in Korea: a nationwide study. Epidemiol Infect. 2013; 141:189–194. PMID: 22717061.
Article6. Doh YS, Kim YS, Jung HJ, et al. Long-term clinical outcome of Clostridium difficile infection in hospitalized patients: a single center study. Intest Res. 2014; 12:299–305. PMID: 25374496.
Article7. Yoon SY, Jung SA, Na SK, et al. What's the clinical features of colitis in elderly people in long-term care facilities? Intest Res. 2015; 13:128–134. PMID: 25931997.
Article8. Mueller S, Saunier K, Hanisch C, et al. Differences in fecal microbiota in different European study populations in relation to age, gender, and country: a cross-sectional study. Appl Environ Microbiol. 2006; 72:1027–1033. PMID: 16461645.
Article9. Barbut F, Monot M, Rousseau A, et al. Rapid diagnosis of Clostridium difficile infection by multiplex real-time PCR. Eur J Clin Microbiol Infect Dis. 2011; 30:1279–1285. PMID: 21487764.
Article10. Berry N, Sewell B, Jafri S, et al. Real-time polymerase chain reaction correlates well with clinical diagnosis of Clostridium difficile infection. J Hosp Infect. 2014; 87:109–114. PMID: 24795170.
Article11. de Jong E, de Jong AS, Bartels CJ, van der Rijt-van den Biggelaar C, Melchers WJ, Sturm PD. Clinical and laboratory evaluation of a real-time PCR for Clostridium difficile toxin A and B genes. Eur J Clin Microbiol Infect Dis. 2012; 31:2219–2225. PMID: 22327373.
Article12. Poutanen SM, Simor AE. Clostridium difficile-associated diarrhea in adults. CMAJ. 2006; 171:51–58.
Article13. Sewell B, Rees E, Thomas I, Ch’ng CL, Isaac M, Berry N. Cost and impact on patient length of stay of rapid molecular testing for Clostridium difficile. Infect Dis Ther. 2014; 3:281–293. PMID: 25183400.
Article14. Vaishnavi C. Clinical spectrum & pathogenesis of Clostridium difficile associated diseases. Indian J Med Res. 2010; 131:487–499. PMID: 20424299.15. Goudarzi M, Seyedjavadi SS, Goudarzi H, Mehdizadeh Aghdam E, Nazeri S. Clostridium difficile infection: epidemiology, pathogenesis, risk factors, and therapeutic options. Scientifica (Cairo). 2014; 2014:916826. PMID: 24991448.16. Warny M, Pepin J, Fang A, et al. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet. 2005; 366:1079–1084. PMID: 16182895.
Article17. Tae CH, Jung SA, Song HJ, et al. The first case of antibiotic-associated colitis by Clostridium difficile PCR ribotype 027 in Korea. J Korean Med Sci. 2009; 24:520–524. PMID: 19543521.
Article18. Kim J, Kang JO, Kim H, et al. Epidemiology of Clostridium difficile infections in a tertiary-care hospital in Korea. Clin Microbiol Infect. 2013; 19:521–527. PMID: 22712697.
Article19. Kim H, Lee Y, Moon HW, Lim CS, Lee K, Chong Y. Emergence of Clostridium difficile ribotype 027 in Korea. Korean J Lab Med. 2011; 31:191–196. PMID: 21779194.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evaluation of the Xpert Clostridium difficile Assay for the Diagnosis of Clostridium difficile Infection
- Diagnosis of Clostridium difficile-associated diarrhea
- Clostridium difficile Infection: What's New?
- Evaluation of Rapid Assay (Tox A/B Quik Chek) for the Detection of Clostridium difficile Toxins A and B
- Comparison and Evaluation of Diagnostic Assays for Clostridium difficile Infection