Nat Prod Sci.
2017 Dec;23(4):306-309. 10.20307/nps.2017.23.4.306.
Microbial Transformation of Two Prenylated Naringenins
- Affiliations
-
- 1College of Pharmacy and Research Institute of Drug Development, Chonnam National University,Gwangju 61186, Republic of Korea. islee@chonnam.ac.kr
- KMID: 2401585
- DOI: http://doi.org/10.20307/nps.2017.23.4.306
Abstract
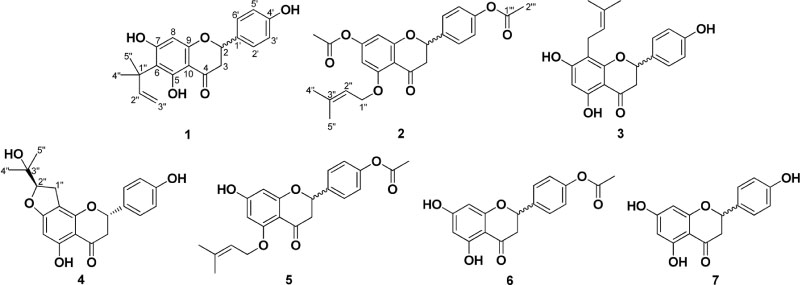
- Microbial transformation of (±)-6-(1,1-dimethylallyl)naringenin (6-DMAN, 1) and (±)-5-(O-prenyl) naringenin-4"²,7-diacetate (5-O-PN, 2) was performed by using fungi. Scale-up fermentation studies with Mucor hiemalis, Cunninghamella elegans var. elegans, and Penicillium chrysogenum led to the isolation of five microbial metabolites. Chemical structures of the metabolites were determined by spectral analyses as (±)-8-prenylnaringenin (3), (2S)-5,4"²-dihydroxy-7,8-[(R)-2-(1-hydroxy-1-methylethyl)-2,3-dihydrofurano]flavanone (4), (±)-5-(O-prenyl)naringenin-4"²-acetate (5), (±)-naringenin-4"²-acetate (6), and (±)-naringenin (7), of which 5 was identified as a new compound.
Keyword
Figure
Reference
-
1. Sumathi R, Tamizharasi S, Sivakumar T. Int J Curr Adv Res. 2015; 4:234–236.2. Ruh MF, Zacharewski T, Connor K, Howell J, Chen I, Safe S. Biochem Pharmacol. 1995; 50:1485–1493.3. Zierau O, Gester S, Schwab P, Metz P, Kolba S, Wulf M, Vollmer G. Planta Med. 2002; 68:449–451.4. Meiyanto E, Hermawan A, Anindyajati . Asian Pac J Cancer Prev. 2012; 13:427–436.5. Assini JM, Mulvihill EE, Huff MW. Curr Opin Lipidol. 2013; 24:34–40.6. Zierau O, Hamann J, Tischer S, Schwab P, Metz P, Vollmer G, Gutzeit HO, Scholz S. Biochem Biophys Res Commun. 2005; 326:909–916.7. Seo EK, Silva GL, Chai HB, Chagwedera TE, Farnsworth NR, Cordell GA, Pezzuto JM, Kinghorn AD. Phytochemistry. 1997; 45:509–515.8. Zierau O, Morrissey C, Watson RWG, Schwab P, Kolba S, Metz P, Vollmer G. Planta Med. 2003; 69:856–858.9. Tokalov SV, Henker Y, Schwab P, Metz P, Gutzeit HO. Pharmacology. 2004; 71:46–56.10. Clark AM, McChesney JD, Hufford CD. Med Res Rev. 1985; 5:231–253.
Article11. Han F, Lee IS. Phytochem Lett. 2016; 18:136–139.12. Han F, Lee IS. Nat Prod Res. 2017; 31:883–889.13. Gester S, Metz P, Zierau O, Vollmer G. Tetrahedron. 2001; 57:1015–1018.14. Kim HJ, Kim SH, Kang BY, Lee IS. Arch Pharm Res. 2008; 31:1241–1246.15. Tahara S, Ingham JL, Mizutani J. Agric Biol Chem. 1987; 51:211–216.16. Jang DS, Cuendet M, Hawthorne ME, Kardono LBS, Kawanishi K, Fong HHS, Mehta RG, Pezzuto JM, Kinghorn AD. Phytochemistry. 2002; 61:867–872.17. Kyriakou E, Primikyri A, Charisiadis P, Katsoura M, Gerothanassis IP, Stamatis H, Tzakos AG. Org Biomol Chem. 2012; 10:1739–1742.18. Maltese F, Erkelens C, van der Kooy F, Choi YH, Verpoorte R. Food Chem. 2009; 116:575–579.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Stereoselective Microbial Hydroxylation of Progestin, Norethisterone by Using Aspergillus niger and Penicillium citrinum
- Data transformation: a focus on the interpretation
- New Production of Antibacterial Polycyclic Quinazoline Alkaloid, Thielaviazoline, from Anthranilic Acid by the Marine-Mudflat-Derived Fungus Thielavia sp.
- Bioprospecting Potential of the Soil Metagenome: Novel Enzymes and Bioactivities
- Osteosarcomatous Transformation in Mazabraud Syndrome: A Case Report