Nat Prod Sci.
2017 Dec;23(4):291-298. 10.20307/nps.2017.23.4.291.
Cytotoxicity and Structure Activity Relationship of Dammarane-Type Triterpenoids from the Bark of Aglaia elliptica against P-388 Murine Leukemia Cells
- Affiliations
-
- 1Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Padjadjaran, Jatinangor 45363, Indonesia. unang.supratman@unpad.ac.id
- 2Central Laboratory of Universitas Padjadjaran, Jatinangor 45363, Indonesia.
- 3Department of Pediatric Dentistry, Faculty of Dentistry, Universitas Padjadjaran, Jatinangor 45363, Indonesia.
- 4Department of Food, Life, and Environmental Science, Faculty of Agriculture, Yamagata University, Tsuruoka, Yamagata 997-8555, Japan.
- KMID: 2401583
- DOI: http://doi.org/10.20307/nps.2017.23.4.291
Abstract
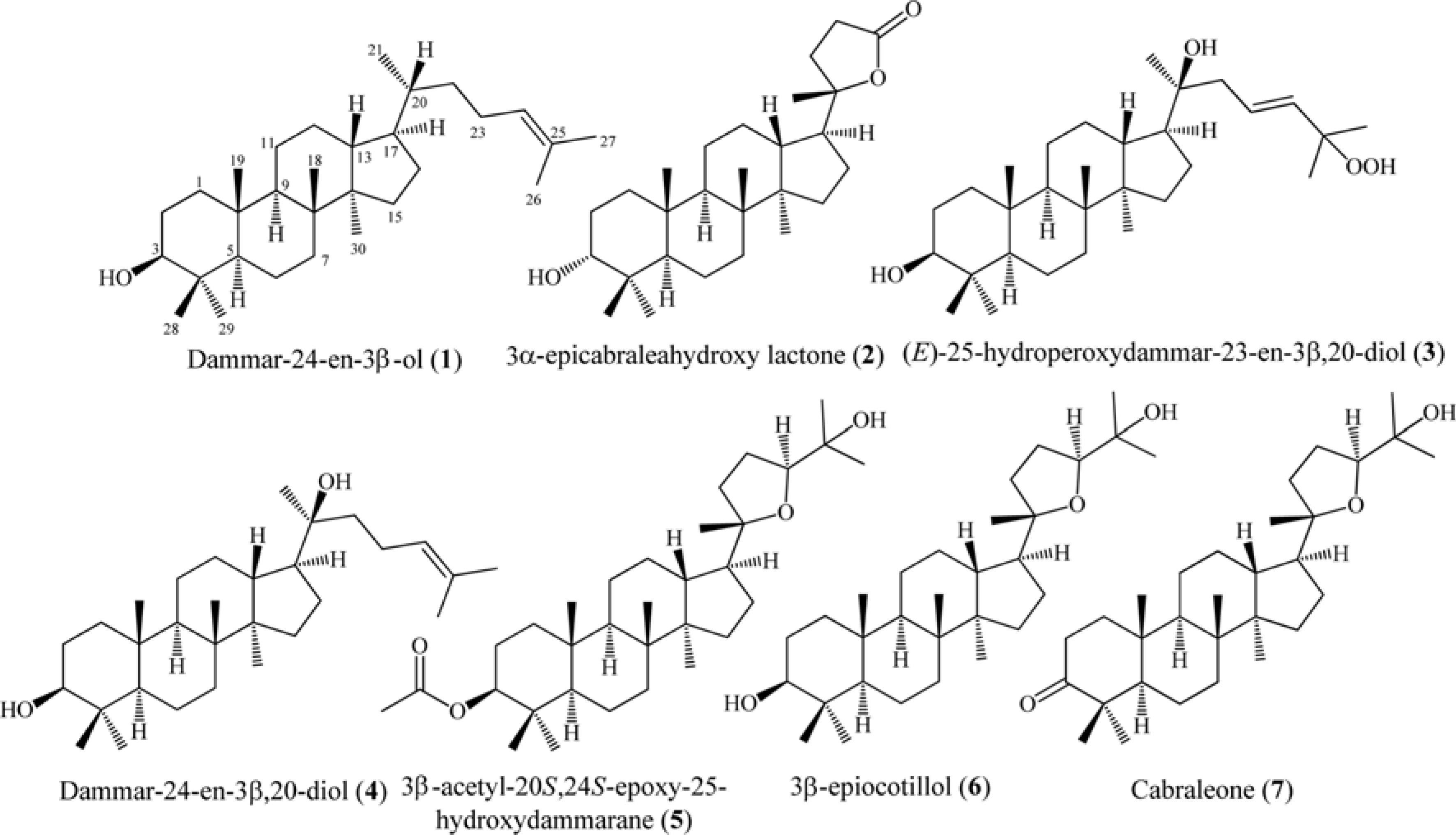
- Six dammarane-type triterpenoids, dammar-24-en-3β-ol (1), 3β-epicabraleahydroxy lactone (2), (E)-25-hydroperoxydammar-23-en-3β,20-diol (3), dammar-24-en-3β,20-diol (4), 3β-acetyl-20S,24S-epoxy-25-hydroxydammarane (5), and 3β-epiocotillol (6) were isolated from the methanolic extract of the bark of Aglaia elliptica. The chemical structure were identified on the basis of spectroscopic evidence and by comparison with those spectra previously reported. Compounds 1 - 6 were isolated first time from this plant. Compounds 1 - 6, along with a known synthetic analog, cabraleone (7) were evaluated their cytotoxic activity against P-388 murine leukimia cells in vitro. Among those compounds 3β-acetyl-20S,24S-epoxy-25-hydroxydammarane (5) showed strongest cytotoxic activity with ICâ‚…â‚€ value of 8.02 ± 0.06 µM.
MeSH Terms
Figure
Reference
-
References
(1). Pannell C. M.Taxonomic monograph of the genus Aglaia lour (Meliaceae): Kew Bulletin Additional Series XVI; HMSO; Richmond,. 1992. 359–362.(2). Harneti D.., Tjokronegoro R.., Safari A.., Supratman U.., Loong X.., Mukhtar M. R.., Mohamad K.., Awang K.., Hayashi H.Phytochem. Lett. 2012. 5:496–499.(3). Muellner A. N.., Samuel R.., Chase M. W.., Pannell C. M.., Greger H.Am. J. Bot. 2005. 92:534–543.(4). Cui B.., Chai H.., Santisuk T.., Reutrakul V.., Farnsworth N. R.., Cordell G. A.., Pezzuto J. M.., Kinghom A. D.Tetrahedron. 1997. 53:17625–17632.(5). Ishibashi F.., Satasook C.., Ismant M. B.., Towers G. H. N.Phytochemistry. 1993. 32:307–310. http://www.sciencedirect.com/science/article/pii/S0031942200949860.(6). Nugroho B. W.., Edrada R. A.., Wray V.., Witte L.., Bringmann G.., Gehling M.., Proksch P.Phytochemistry. 1999. 51:367–376.(7). Wu T. S.., Liou M. J.., Kuoh C. S.., Teng C. M.., Nagao T.., Lee K. H. J.Nat. Prod. 1997. 60:606–608.(8). Esimone C. O.., Eck G.., Nworu C. S.., Hoffmann D.., Uberla K.., Proksch P.Phytomedicine. 2010. 17:540–547.(9). Sianturi J.., Purnamasari M.., Darwati ., Harneti D.., Mayanti T.., Supratman U.., Awang K.., Hayashi H.Phytochem. Lett. 2015. 13:297–301.(10). Joycharat N.., Plodpai P.., Panthong K.., Yingyongnarongkul B.., Voravuthikunchai S. P.Can. J. Chem. 2010. 88:937–944.(11). Liu S.., Liu S. B.., Zuo W. J.., Guo Z. K.., Mei W. L.., Dai H. F.Fitoterapia. 2014. 92:93–99.(12). Yodsaoue O.., Sonprasit J.., Karalai C.., Ponglimanont C.., Tewtrakul S.., Chantrapromma S.Phytochemistry. 2012. 76:83–91.(13). Cai X. H.., Wang Y. Y.., Zhao P. J.., Li Y.., Luo X. D.Phytochemistry. 2010. 71:1020–1024.(14). Roux D.., Martin. M.., Adeline M.., Sevenet T.., Hadi A. H.., Pais M.Phytochemistry. 1998. 49:1745–1748.(15). Xie B. J.., Yang S. P.., Chen H. D.., Yue J. M. J.Nat. Prod. 2007. 70:1532–1535.(16). Zhang F.., Wang J. S.., Gu Y. C.., Kong L. Y. J.Nat. Prod. 2010. 73:2042–2046.(17). Mohamad K.., Sévenet T.., Dumontet V.., Pa s M.., Tri M. V.., i Hadi H.., Awang K.., Martin M. T.Phytochemistry. 1999. 51:1031–1037.(18). Awang K.., Loong X. M.., Leong K. H.., Supratman U.., Litaudon M.., Mukhtar M. R.., Mohamad K.Fitoterapia. 2012. 83:1391–1395.(19). Mohamad K.., Martin M. T.., Najdar H.., Gaspard C.., Sevenet T.., Awang K.., Hadi H.., Pais M. J.Nat. Prod. 1999. 62:868–872.(20). Farabi K.., Harneti D.., Nurlelasari; Maharani R.., Hidayat A. T.., Awang K.., Supratman U.., Shiono Y.Phytochem. Lett. 2017. 21:211–215.(21). Breitmaier E.Structure elucidation by NMR in organic chemistry; John Wiley & Sons,. 2002. , London.(22). Cysne Jde B.., Braz-Filho R.., Assuncao M. V.., Uchoa D. E.., Silveira E. R.., Pessoa O. D.Magn. Reson. Chem. 2006. 44:641–643.(23). Phongmaykin J.., Kumamoto T.., Ishikawa T.., Suttisri R.., Saifah E.Arch. Pharm. Res. 2008. 31:21–27.(24). Zhang F.., Wang J. S.., Gu Y. C.., Kong L. Y. J.Nat. Prod. 2010. 73:2042–2046.(25). Sahidin ., Hakim E. H.., Juliawaty L. D.., Syah Y. M.., bin Din L.., Ghisalberti E. L.., Latip J.., Said I. M.., Achmad S. A. Z.Naturforsch. C. 2005. 60:723–727.(26). Alley M. C.., Scudiero D. A.., Monks A.., Hursey M. L.., Czerwinski M. J.., Fine D. L.., Abbott B. J.., Mayo J. G.., Shoemaker R. H.., Boyd M. R.Cancer Res. 1988. 48:589–601.(27). Hakim E. H.., Achmad S. A.., Juliawaty L. D.., Makmur L.., Syah Y. M.., Aimi A.., Kitajima M.., Takayama H.., Ghisalberti E. L. J.Nat. Med. 2007. 61:229.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Acronyculatin P, A New Isoprenylated Acetophenone from the Stem Bark of Acronychia pedunculata
- Meliglabrin, A New Flavonol Derivative from the leaves of Melicope glabra (Blume) T.G. Hartley
- A New Cytotoxic Compound from Methanol Extract of Koordersiodendron pinnatum Merr. Leaves
- Diels-Alder Type Adducts from Hairy Root Cultures of Morus macroura
- Cytotoxic Activity and Quantitative Structure Activity Relationships of Arylpropyl Sulfonamides