Nat Prod Sci.
2017 Dec;23(4):286-290. 10.20307/nps.2017.23.4.286.
Anti-inflammatory and Anti-bacterial Effects of Aloe vera MAP against Multidrug-resistant Bacteria
- Affiliations
-
- 1College of Pharmacy, Duksung Women's University, Seoul 01369, Republic of Korea. hsshin@duksung.ac.kr
- KMID: 2401582
- DOI: http://doi.org/10.20307/nps.2017.23.4.286
Abstract
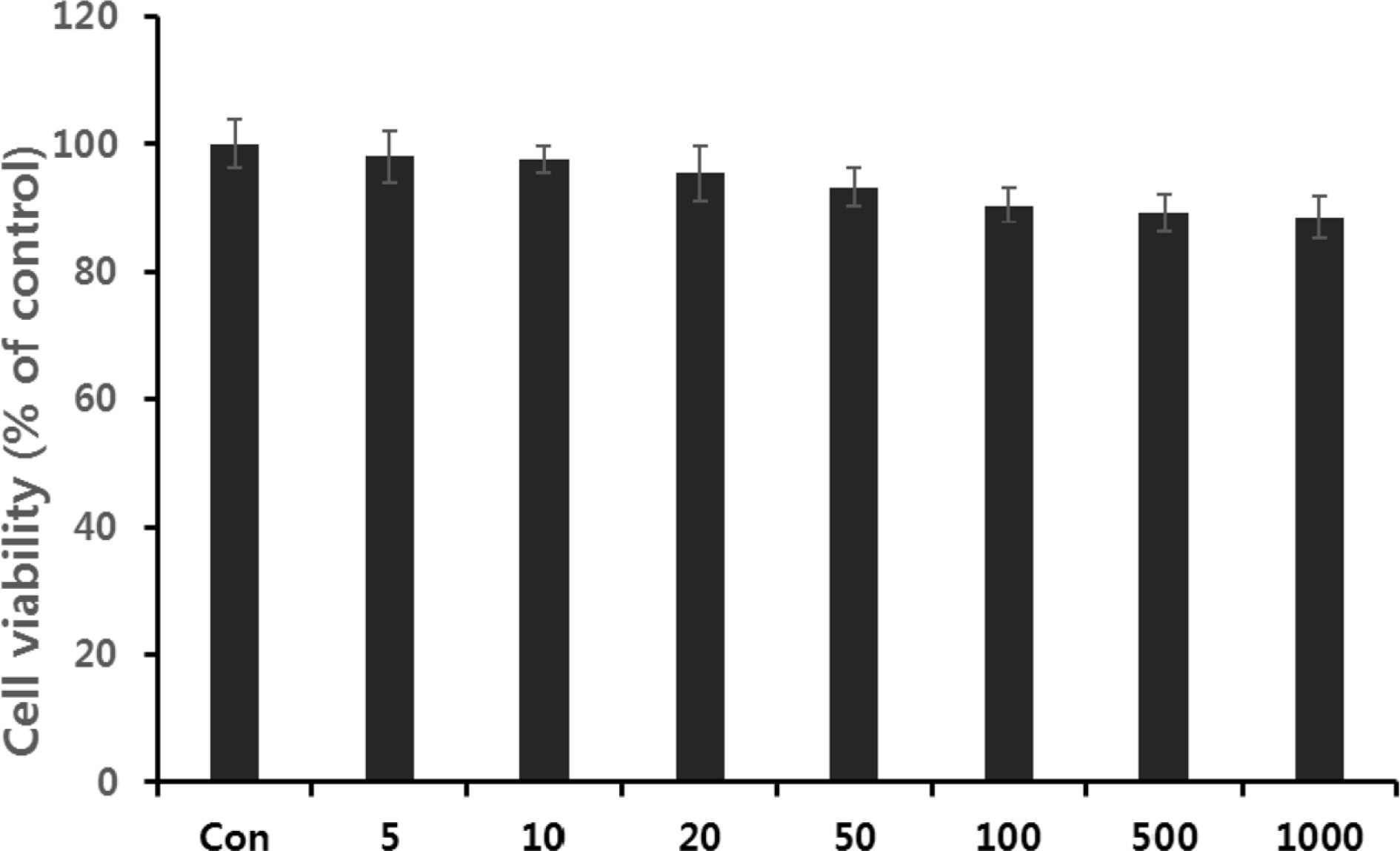
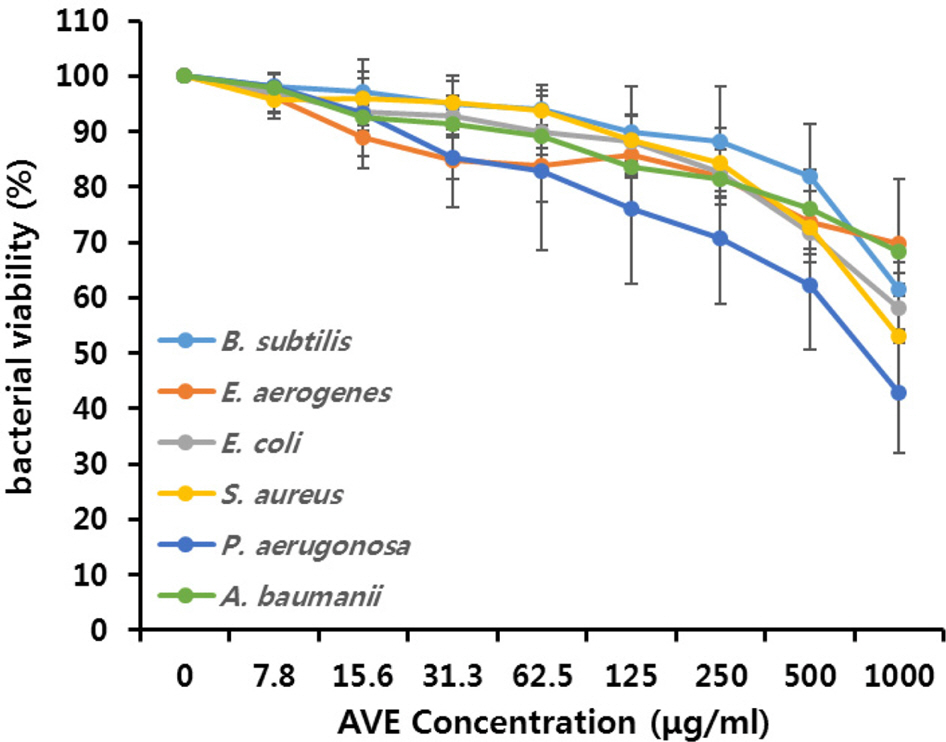
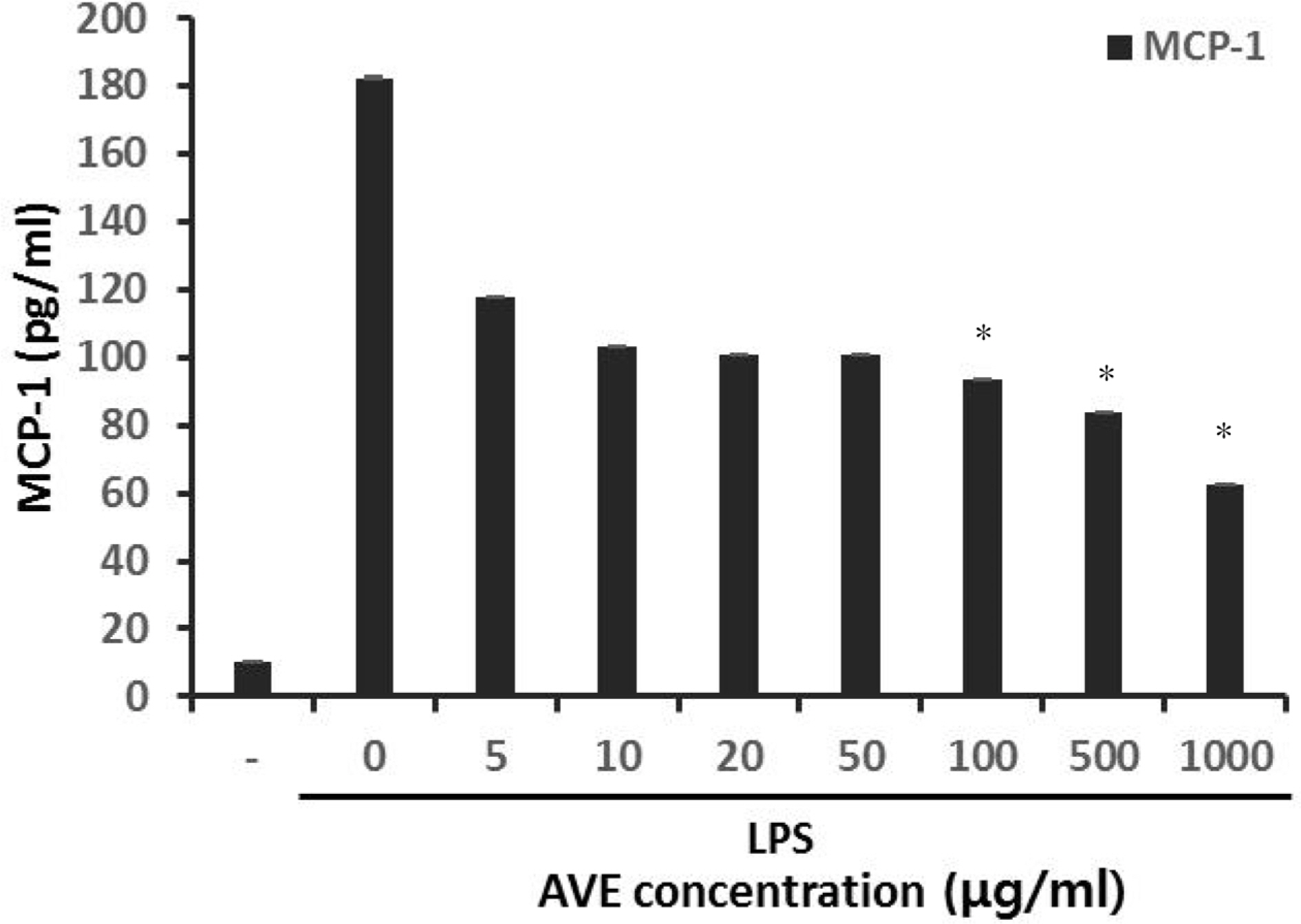
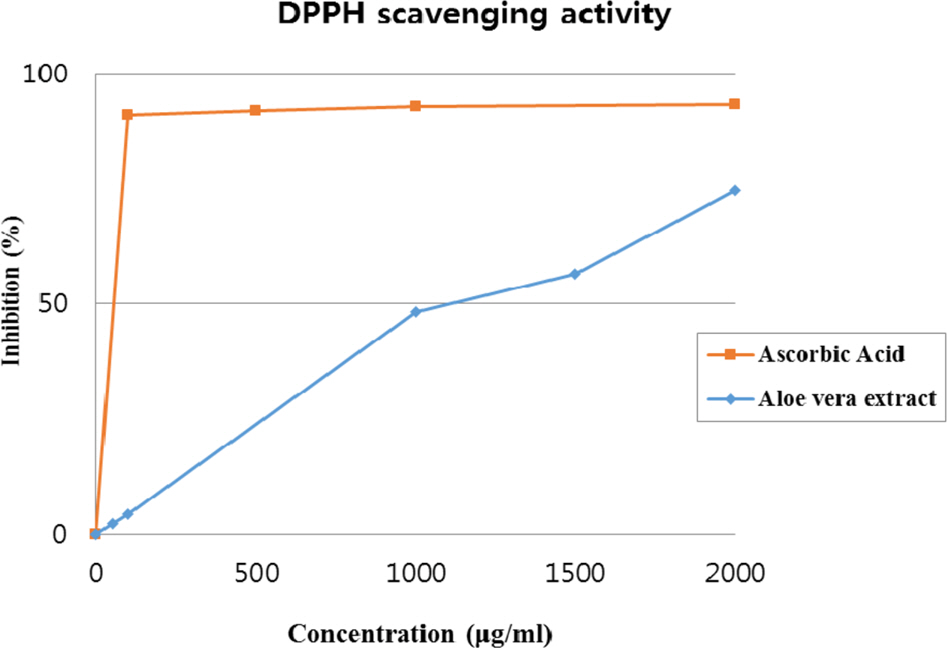
- Multidrug-resistant Acinetobacter baumannii and Pseudomonas aeruginosa are highly dangerous nosocomial pathogens, cause the symptoms of skin infections, pressure sores, sepsis, blood stream and wound infections. Unfortunately, these pathogens are immune to the most common antibiotics, such as, carbapenem, aminoglycoside and fluoroquinolone. Therefore, it is imperative that new and effective antibiotics be developed. In the present study, the antimicrobial effects of Aloe vera MAP (modified Aloe polysaccharide) on Staphylococcus aureus and Bacillus subtilis, Escherichia coli and Enterobacter aerogenes, and clinical Pseudomonas aeruginosa and clinical Acinetobacter baumannii were comprehensibly investigated. Prior to the growth inhibition effect measurement and antibiotic disc diffusion assay on gram-positive and gram-negative bacteria and selected multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii, antimicrobial resistance screening was performed for the multidrug-resistant bacteria obtained from clinical isolates. The results for showed the Aloe vera MAP had a concentration-dependent effect on all of examined bacteria, particularly on Pseudomonas aeruginosa. Anti-inflammatory and anti-oxidant experiments were also performed dose dependently effects to confirm the beneficial physiological effects of Aloe vera MAP.
MeSH Terms
Figure
Reference
-
References
(1). Gaynes R.., Edwards J.R.; Clin. Infect. Dis. 2005. 41:848–854.
Article(2). Doring G.., Horz M.., Ortelt J.., Grupp H.., Wolz C.Epidemiol. Infect. 1993. 110:427–436.(3). Goudarzi M.., Fazeli M.., Azad M.., Seyedjavadi S. S.., Mousavi R.Chemother Res Prac. 2015. 2015:1–5.(4). Falagas M. E.., Koletsi P. K.., Bliziotis I. A. J.Med. Microbiol. 2006. 55:1619–1629.
Article(5). Pankuch G. A.., Seifert H.., Appelbaum P. C.Diagn. Microbiol. Infect. Dis. 2010. 67:191–197.(6). Vidal F.., Mensa J.., Almela M.., Martinez J. A.., Marco F.., Casals C.., Gatell J. M.., Soriano E.., Jimenez de Anta M. T.Arch. Intern. Med. 1996. 156:2121–2126.(7). Kuo L. C.., Teng L. J.., Yu C. J.., Ho S. W.., Hsueh P. R. J.Clin. Microbiol. 2004. 42:1759–1763.(8). Deplano A.., Denis O.., poriel, L. Hocquet D.., Nonhoff, C. Byl B.., Nordmann P.., Vincent J. L.., Struelens M. J. J.Clin. Microbiol. 2005. 43:1198–1204.(9). Ahluwalia B.., Magnusson M.K.; Isaksson, S.; Larsson, F.; Öhman, LJ. Ethnopharmacol. 2016. 179:301–309.(10). Vijayalakshmi D.., Dhandapani R.., Jayaveni S.., Jithendra P. S.., Rose C.., Mandal A. B. J.Ethnopharmacol. 2012. 141:542–546.(11). Rathor N.., Mehta A. K.., Sharma A. K.., Mediratta P. K.., Sharma K. K.Inflammation. 2012. 35:1900–1903.(12). Kim M. U.., Cho Y. J.., Lee S. Y.J Appl. Biol. Chem. 2013. 56:157–163.(13). Habeeb F.., Shakir E.., Bradbury F.., Cameron P.., Taravati M. R.., Drummond A. J.., Gray A. I.., Ferro V. A.Methods. 2007. 42:315–320.(14). Qiu Z.., Jones K.., Wylie M.., Jia Q.., Orndorff S.Planta. Med. 2000. 66:152–156.(15). Im S. A.., Oh S. T.., Song S.., Kim M. R.., Kim D. S.., Woo S. S.., Jo T. H.., Park Y. I.., Lee C. K.Int. Immunopharmacol. 2005. 5:271–279.(16). Lee D. K.., Kim M. J.., Kang J. Y.., Park J. E.., Shin H. S.., Ha N. J.Kor. J. Microbiology. 2013. 49:245–252.(17). Pugh N.., Ross S. A.., ElSohly M. A.., Pasco D. S. J.Agric. Food Chem. 2001. 49:1030–1034.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Therapeutic strategy for the management of multidrug-resistant gram-negative bacterial infections
- Multidrug-Resistant Gram-Positive Bacterial Infections
- Anti-irritant Effect of Aloe Vera Gel Against a Lactic Acid Sting Test Reaction
- Multidrug-resistant bacteria: a national challenge requiring urgent addressal
- A Study on Anti-irritant Effect of Aloe Vera Gel Against the Irritation of Sodium Lauryl Sulfate