Nat Prod Sci.
2017 Dec;23(4):247-252. 10.20307/nps.2017.23.4.247.
Rapid Identification of Methylglyoxal Trapping Constituents from Onion Peels by Pre-column Incubation Method
- Affiliations
-
- 1College of Pharmacy and Institute of Pharmaceutical Science and Technology, Hanyang University, Ansan, Gyeonggi-do 15588, Republic of Korea. chulykim@hanyang.ac.kr
- 2Department of Pharmacognosy, Faculty of Pharmacy, University of Sindh, Jamshoro, Pakistan.
- 3College of Pharmacy, CHA University, Sungnam 13844, Republic of Korea.
- KMID: 2401575
- DOI: http://doi.org/10.20307/nps.2017.23.4.247
Abstract
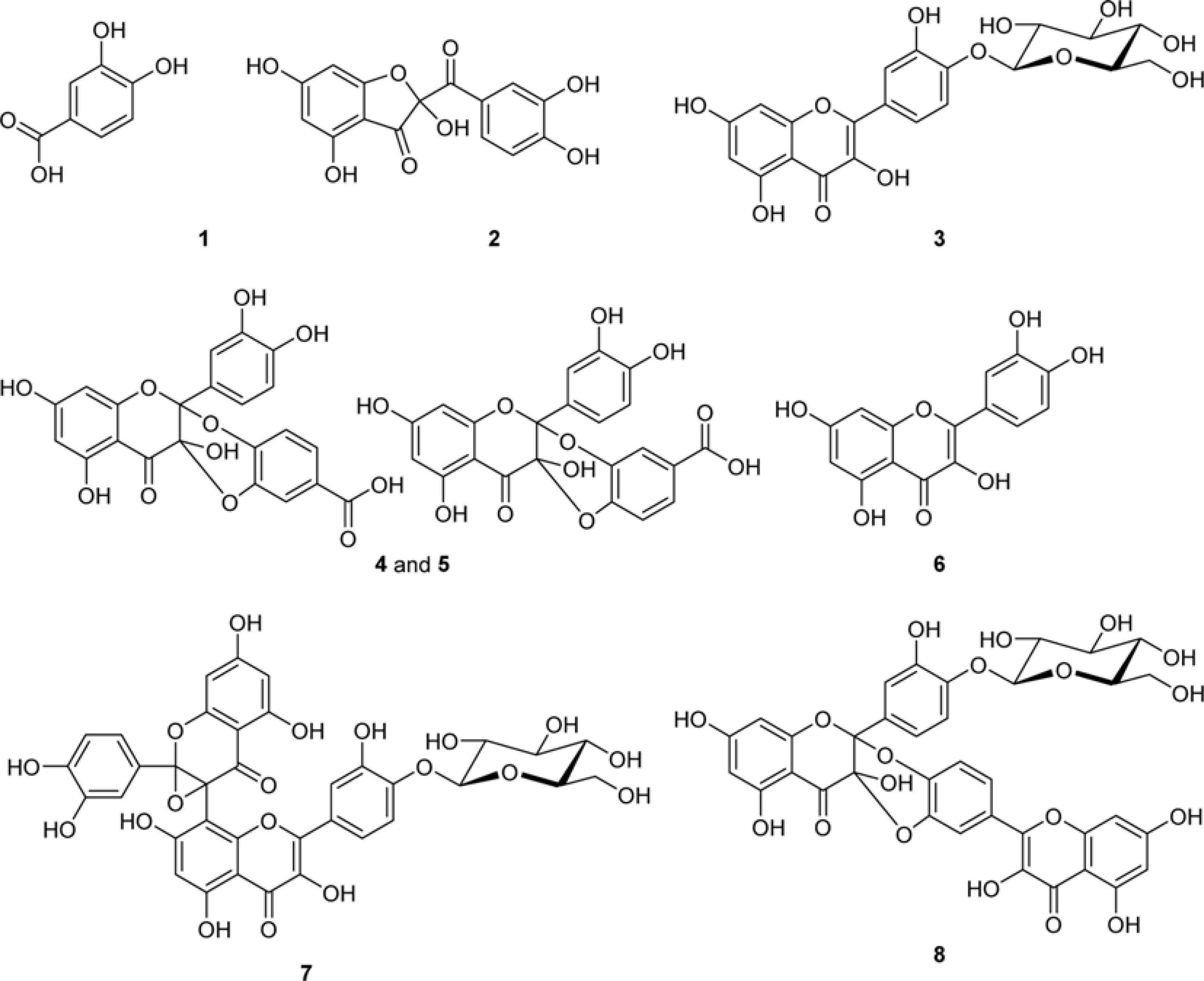
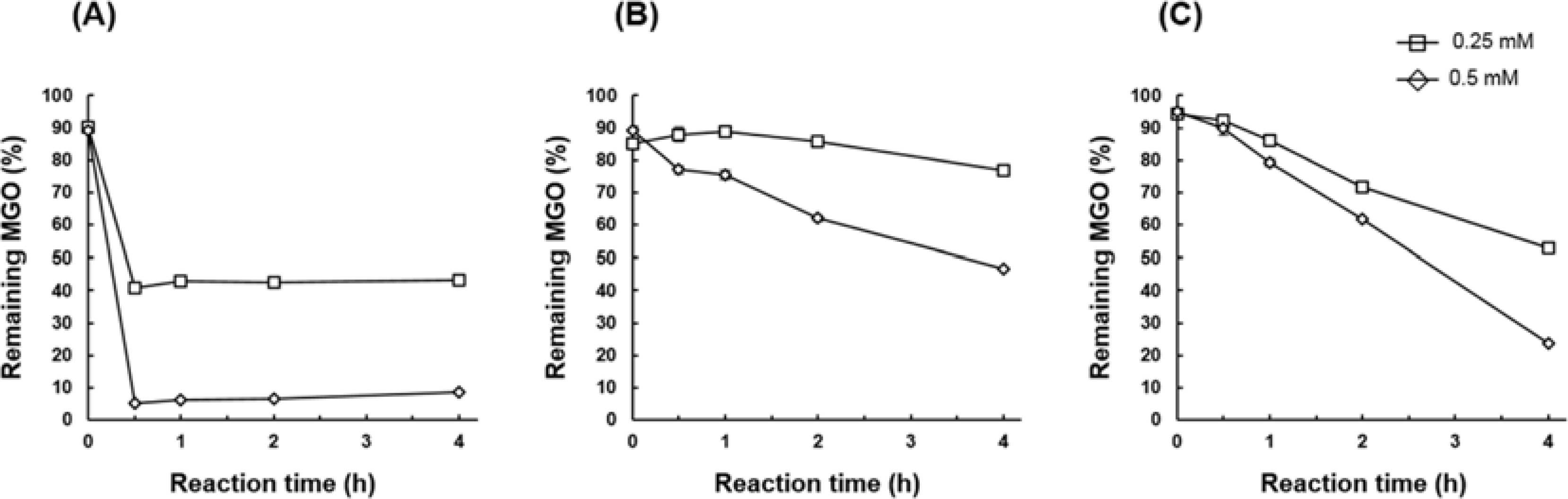
- The methylglyoxal (MGO) trapping constituents from onion (Allium cepa L.) peels were investigated using pre-column incubation of MGO and crude extract followed by HPLC analysis. The peak areas of MGO trapping compounds decreased, and their chemical structures were identified by HPLC-ESI/MS. Among major constituents in outer scale of onion, 2-(3,4-dihydroxybenzoyl)-2,4,6-trihydroxy-3(2H)-benzofuranone (2) was more effective MGO scavenger than quercetin (6) and its 4"²-glucoside, spiraeoside (3). After 1 h incubation, compound 2 trapped over 90% MGO at a concentration of 0.5 mM under physiological conditions, but compounds 3 and 6 scavenged 45%, 16% MGO, respectively. HPLC-ESI/MS showed that compound 2 trapped two molecules of MGO to form a di-MGO adduct and compounds 3 and 6 captured one molecule of MGO to form mono-MGO adducts, and the positions 6 and 8 of the A ring of flavonoids were major active sites for trapping MGO.
MeSH Terms
Figure
Reference
-
References
(1). Ramasamy R.., Yan S. F.., Schmidt A. M.Cell. 2006. 124:258–260.(2). Tang D.., Zhu J. X.., Wu A. G.., Xu Y. H.., Duan T. T.., Zheng Z. G.., Wang R. S.., Li D.., Zhu Q. J.Chromatogr. A. 2013. 1286:102–110.(3). Lv L.., Shao X.., Chen H.., Ho C. T.., Sang S.Chem. Res. Toxico. 2011. 24:579–586.(4). Lv L.., Shao X.., Wang L.., Huang D.., Ho C. T.., Sang S. J.Agric. Food Chem. 2010. 58:2239–2245.(5). Schleicher E. D.., Wagner E.., Nerlich A. G. J.Clin. Invest. 1997. 99:457–468.(6). Yoon S. R.., Shim S. M.LWT-Food Sci. Technol. 2015. 61:158–163.(7). Shao X.., Bai N.., He K.., Ho C. T.., Yang C. S.., Sang S.Chem. Res. Toxicol. 2008. 21:2042–2050.(8). Li X.., Zheng T.., Sang S.., Lv L. J.Agric. Food Chem. 2014. 62:12152–12158.(9). Yang B. N.., Choi E. H.., Shim S. M.Appl. Biol. Chem. 2017. 60:57–62.(10). Totlani V. M.., Peterson D. G. J.Agric. Food Chem. 2006. 54:7311–7318.(11). Lo C. Y.., Li S.., Tan D.., Pan M. H.., Sang S.., Ho C. T.Mol. Nutr. Food Res. 2006. 50:1118–1128.(12). Hu T. Y.., Liu C. L.., Chyau C. C.., Hu M. L. J.Agric. Food Chem. 2012. 60:8190–8196.(13). Peng X.., Cheng K. W.., Ma J.., Chen B.., Ho C. T.., Lo C.., Chen F.., Wang M. J.Agric. Food Chem. 2008. 56:1907–1911.(14). Chen X. Y.., Huang I. M.., Hwang L. S.., Ho C. T.., Li S.., Lo C. Y. J.Funct. Foods. 2014. 8:259–268.(15). Zhu Y.., Zhao Y.., Wang P.., Ahmedna M.., Sang S.Chem. Res. Toxicol. 2015. 28:1842–1849.(16). Ly T. N.., Hazama C.., Shimoyamada M.., Ando H.., Kato K.., Yamauchi R. J.Agric. Food Chem. 2005. 53:8183–8189.(17). Xue Y. L.., Ahiko T.., Miyakawa T.., Amino H.., Hu F.., Furihata K.., Kita K.., Shirasawa T.., Sawano Y.., Tanokura M. J.Agric. Food Chem. 2011. 59:5927–5934.(19). Shao X.., Chen H.., Zhu Y.., Sedighi R.., Ho C. T.., Sang S. J.Agric. Food Chem. 2014. 62:3202–3210.(20). Wang P.., Chen H.., Sang S.Chem. Res. Toxicol. 2016. 29:406–414.(21). Zhu D.., Wang L.., Zhou Q.., Yan S.., Li Z.., Sheng J.., Zhang W.Mol. Nutr. Food Res. 2014. 58:2249–2260.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Trapping of Methylglyoxal by Sieboldin from Malus baccata L. and Identification of Sieboldin-Methylglyoxal Adducts Forms
- Usefulness of column agglutination test for irregular antibody screening and identification
- Antioxidant Effects on various solvent extracts from Onion Peel and Onion Flesh
- Same-Day Identification and Antimicrobial Susceptibility Testing of Bacteria in Positive Blood Culture Broths Using Short-Term Incubation on Solid Medium with the MicroFlex LT, Vitek-MS, and Vitek2 Systems
- Aerobic Exercise Ameliorates Muscle Atrophy Induced by Methylglyoxal via Increasing Gastrocnemius and Extensor Digitorum Longus Muscle Sensitivity