Pediatr Infect Vaccine.
2017 Dec;24(3):134-140. 10.14776/piv.2017.24.3.134.
The First Newborn Screening Study of T-Cell Receptor Excision Circle and κ- Deleting Recombination Excision Circle for Severe Combined Immunodeficiency in Korea: A Pilot Study
- Affiliations
-
- 1Department of Pediatrics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, the Republic of Korea. yaejeankim@skku.edu
- 2Center for Pediatric Cancer, National Cancer Center, Goyang, the Republic of Korea.
- 3Department of Pediatrics, Myongji Hospital, Seonam University College of Medicine, Goyang, the Republic of Korea.
- 4Department of Pediatrics, Cheil General Hospital & Women's Healthcare Center, Dankook University College of Medicine, Seoul, the Republic of Korea.
- KMID: 2401387
- DOI: http://doi.org/10.14776/piv.2017.24.3.134
Abstract
- PURPOSE
Severe combined immunodeficiency (SCID) is the most serious form of primary immunodeficiency. Infants with SCID are susceptible to life-threatening infections. To establish newborn screening for SCID in Korea, we performed a screening test for T-cell receptor excision circle (TREC) and κ-deleting recombination excision circle (KREC) in neonates and investigated the awareness of SCID among their parents.
METHODS
Collections of dried blood spots from neonates and parent surveys were performed at the Samsung Medical Center and Cheil General Hospital & Women's Healthcare Center in Korea. The amplification crossing point (Cp) value <37.0 was defined as TREC/KRECpositive based on cutoff values from measuring multiplex real-time polymerase chain reaction. A Cp value >39.0 was defined as negative.
RESULTS
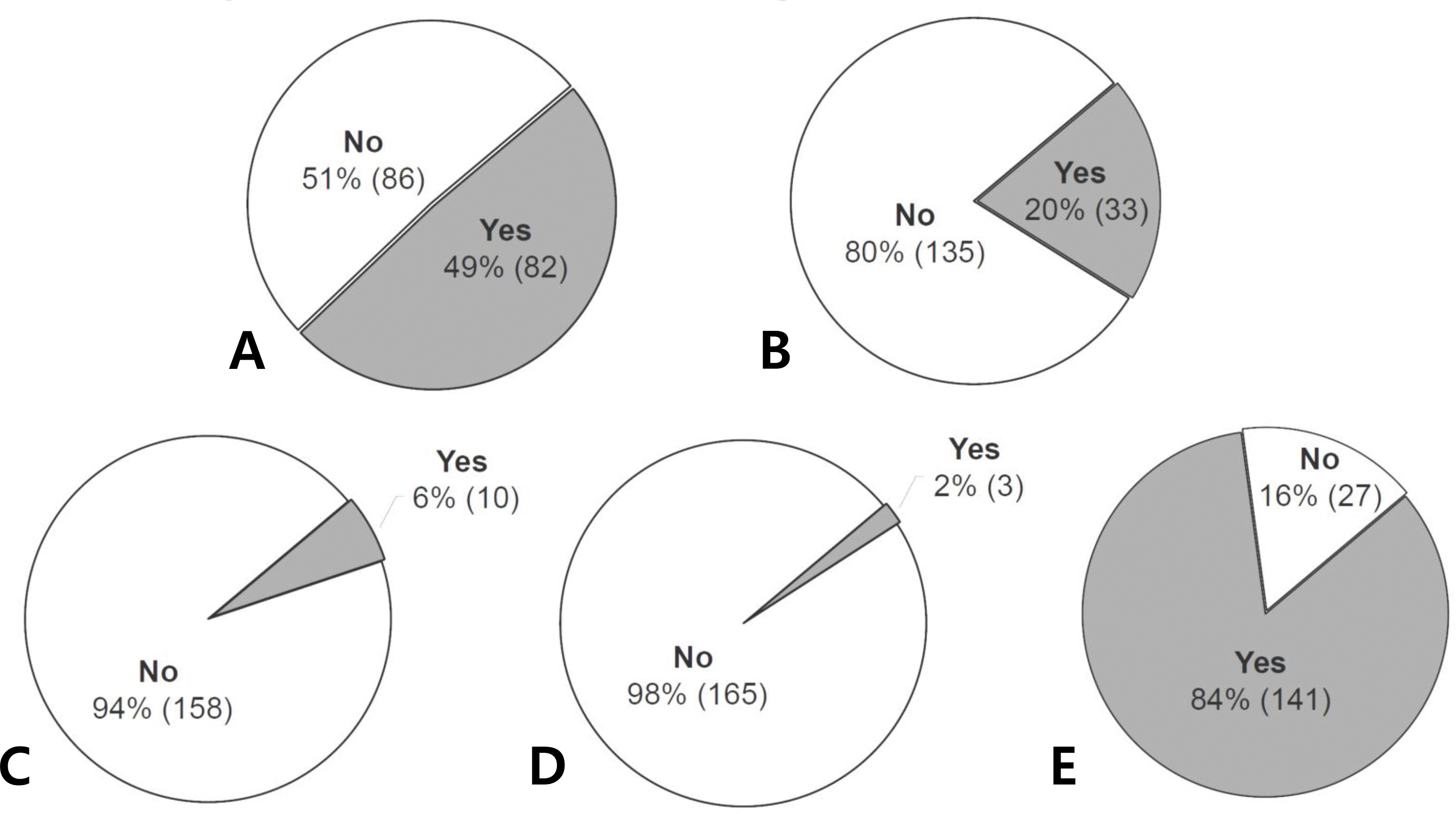
For TREC/KREC screening, 141 neonates were enrolled; 63 (44.7%) were male. One hundred forty neonates (99.3%) had positive TREC/KREC results at the time of the initial test; 82.3% and 75.9% were positive and 17.0% and 23.4% were weakly positive for TREC and KREC, respectively. In one neonate (0.7%), the initial TREC/KREC test result was negative. However, repeated tests obtained and confirmed a positive result. For an awareness survey, 168 parents were engaged. Only 2% of parents (3/168) knew that the newborn screening test for SCID had been introduced and performed in other countries. Eighty-four percent of parents (141/168) replied that nationwide newborn SCID screening should be performed in Korean newborns.
CONCLUSIONS
In this study, newborn SCID screening was performed along with assessment of public awareness of the SCID test in Korea. The study results showed that newborn SCID screening can be readily applied for clinical use at a relatively low cost in Korea.
MeSH Terms
-
Delivery of Health Care
Hospitals, General
Humans
Infant
Infant, Newborn*
Korea*
Male
Mass Screening*
Neonatal Screening
Parents
Pilot Projects*
Real-Time Polymerase Chain Reaction
Receptors, Antigen, T-Cell*
Recombination, Genetic*
Severe Combined Immunodeficiency*
Surveys and Questionnaires
T-Lymphocytes*
Receptors, Antigen, T-Cell
Figure
Reference
-
1. Buckley RH, Schiff SE, Schiff RI, Markert L, Williams LW, Roberts IL, et al. Hematopoietic stem—cell transplantation for the treatment of severe combined immunodeficiency. N Engl I Med. 1999; 340:508–16.
Article2. Brown L, Xu—Bayford I, Allwood Z, Slatter M, Cant A, Davies EG, et al. Neonatal diagnosis of severe combined immunodeficiency leads to significantly improved survival outcome: the case for newborn screening. Blood. 2011; 117:3243–6.
Article3. Pai SY, Logan BR, Griffith LM, Buckley RH, Parrott RE, Dvorak CC, et al. Transplantation outcomes for severe combined immunodeficiency, 2000—2009. N Engl I Med. 2014; 371:434–46.
Article4. Immune Deficiency Foundation (IDF). IDF SCID newborn screening campaign [Internet]. Towson: IDF;c2017. [cited 2017 Nov 7]. Available from:. https://primaryimmune. org.5. GOV.UK. Population screening programmes [Internet]. GOV.UK.;. 2017. [cited 2017 Nov 7]. Available from:. https://www.gov.uk/topic/population—screening—programmes/newborn—blood—spot.6. van Zelm MC, van der Burg M, Langerak AW, van Dongen II. PID comes full circle: applications of V(D) I recombination excision circles in research, diagnostics and newborn screening of primary immunodeficiency disorders. Front Immun01. 2011; 2:12.
Article7. Borte S, V0n Dobeln U, Fasth A, Wang N, Ianzi M, Wini-arski I, et al. Neonatal screening for severe primary immunodeficiency diseases using high—throughput triplex real—time PCR.B100d. 2012; 119:2552–5.8. Kwan A, Abraham RS, Currier R, Brewer A, Andruszewski K, Abbott IK, et al. Newborn screening for severe combined immunodeficiency in 11 screening programs in the United States. IAMA. 2014; 312:729–38.9. Bousfiha A, Jeddane L, Al-Herz W, Ailal F, Casanova IL, Chatila T, et al. The 2015 IUIS phenotypic classification for primary immunodeficiencies. I Clin Immunol. 2015; 35:727–38.
Article10. Chien YH, Chiang SC, Chang KL, Yu HH, Lee WI, Tsai LP, et al. Incidence of severe combined immunodeficiency through newborn screening in a Chinese population. J Formos Med Assoc. 2015; 114:12–6.
Article11. Rhim IVV, Kim KH, Kim DS, Kim BS, Kim IS, Kim CH, et al. Prevalence of primary immunodeficiency in Korea. I Korean Med 5C1. 2012; 27:788–93.
Article12. Centerwall WR, Chinnock RF, Pusavat A. Phenylketonuria: screening programs and testing methods. Am I Public Health Nations Health. 1960; 50:1667–77.
Article13. Puck IM. The case for newborn screening for severe combined immunodeficiency and related disorders. Ann N Y Acad 5C1. 2011. 12462108–17.
Article14. McGhee SA, Stiehm ER, McCabe ER. Potential costs and benefits of newborn screening for severe combined immunodeficiency. I Pediatr. 2005; 147:603–8.
Article15. Lee DH. Newborn screening of inherited metabolic disease in Korea. Korean I Pediatr. 2006; 49:1125–39.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Modern diagnostic capabilities of neonatal screening for primary immunodeficiencies in newborns
- A study on the normal dental arch form of Korean adult
- Circle within a Circle: Unique Appearance of Coronary Air Embolism on OCT Imaging
- The Morphologic Study of Willisian Circle
- Usefulness of Computed Tomographic Angiography in the Detection and Evaluation of Aneurysms of the Circle of Willis