Immune Netw.
2017 Oct;17(5):343-351. 10.4110/in.2017.17.5.343.
Plasmacytoid Dendritic Cells Contribute to the Protective Immunity Induced by Intranasal Treatment with Fc-fused Interleukin-7 against Lethal Influenza Virus Infection
- Affiliations
-
- 1Division of Integrative Biosciences and Biotechnology (IBB), Pohang University of Science and Technology (POSTECH), Pohang 37673, Korea. sw_lee@postech.ac.kr
- 2Research Institute, Genexine Inc., Korea Bio Park, Seongnam 13488, Korea.
- 3Department of Life Sciences, Pohang University of Science and Technology (POSTECH), Pohang 37673, Korea.
- KMID: 2400639
- DOI: http://doi.org/10.4110/in.2017.17.5.343
Abstract
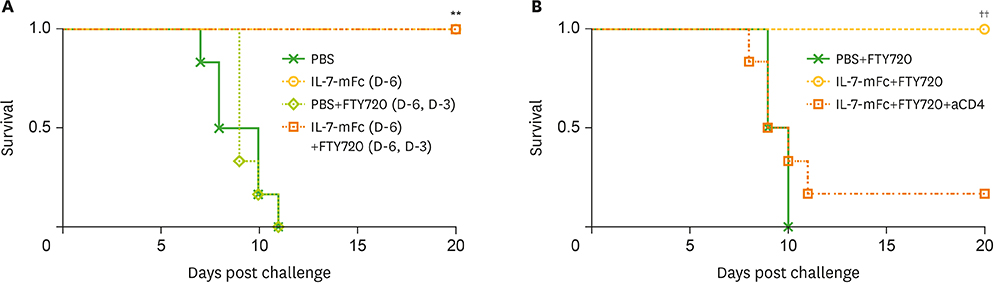
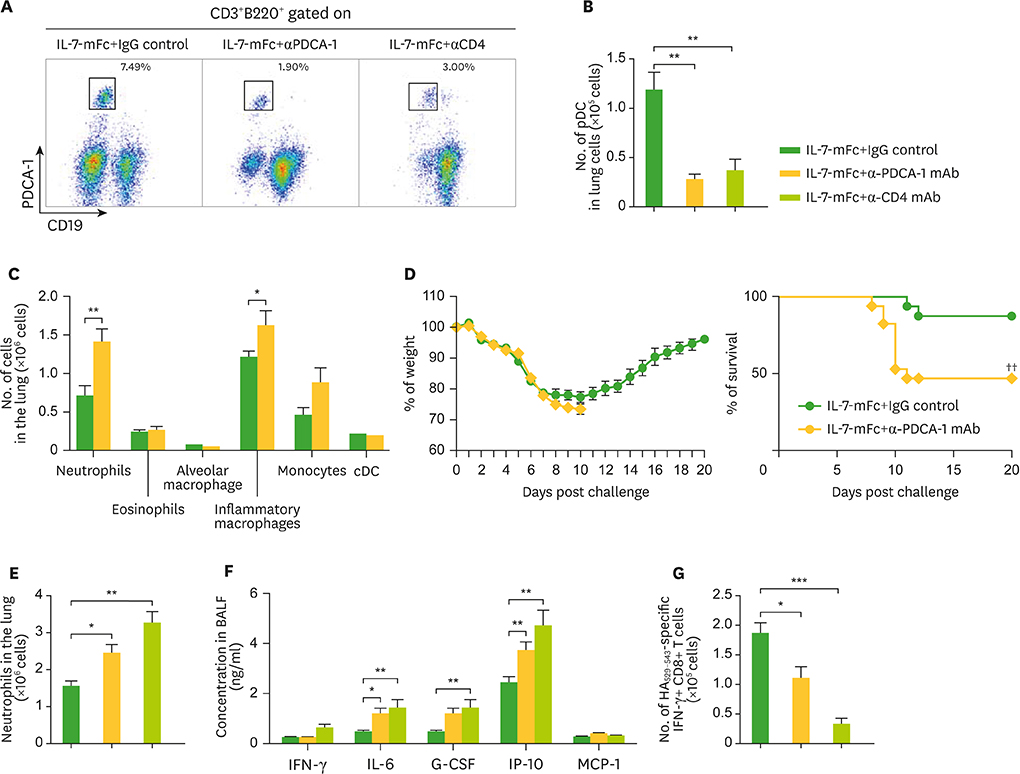
- Developing a novel vaccine that can be applied against multiple strains of influenza virus is of utmost importance to human health. Previously, we demonstrated that the intranasal introduction of Fc-fused IL-7 (IL-7-mFc), a long-acting cytokine fusion protein, confers long-lasting prophylaxis against multiple strains of influenza A virus (IAV) by inducing the development of lung-resident memory-like T cells, called T(RM)-like cells. Here, we further investigated the mechanisms of IL-7-mFc-mediated protective immunity to IAVs. First, we found that IL-7-mFc treatment augments the accumulation of pulmonary T cells in 2 ways: recruiting blood circulating T cells into the lung and expanding T cells at the lung parenchyma. Second, the blockade of T cell migration from the lymph nodes (LNs) with FTY720 treatment was not required for mounting the protective immunity to IAV with IL-7-mFc, suggesting a more important role of IL-7 in T cells in the lungs. Third, IL-7-mFc treatment also recruited various innate immune cells into the lungs. Among these cells, plasmacytoid dendritic cells (pDCs) play an important role in IL-7-mFc-mediated protective immunity through reducing the immunopathology and increasing IAV-specific cytotoxic T lymphocyte (CTL) responses. In summary, our results show that intranasal treatment with IL-7-mFc modulates pulmonary immune responses to IAV, affecting both innate and adaptive immune cells.
MeSH Terms
Figure
Reference
-
1. Cowling BJ, Jin L, Lau EH, Liao Q, Wu P, Jiang H, Tsang TK, Zheng J, Fang VJ, Chang Z, et al. Comparative epidemiology of human infections with avian influenza A H7N9 and H5N1 viruses in China: a population-based study of laboratory-confirmed cases. Lancet. 2013; 382:129–137.
Article2. Bouvier NM, Palese P. The biology of influenza viruses. Vaccine. 2008; 26:Suppl 4. D49–D53.
Article3. Hurt AC, Ernest J, Deng YM, Iannello P, Besselaar TG, Birch C, Buchy P, Chittaganpitch M, Chiu SC, Dwyer D, et al. Emergence and spread of oseltamivir-resistant A(H1N1) influenza viruses in Oceania, South East Asia and South Africa. Antiviral Res. 2009; 83:90–93.
Article4. Stephenson I, Nicholson KG. Influenza: vaccination and treatment. Eur Respir J. 2001; 17:1282–1293.
Article5. Jegaskanda S, Reading PC, Kent SJ. Influenza-specific antibody-dependent cellular cytotoxicity: toward a universal influenza vaccine. J Immunol. 2014; 193:469–475.
Article6. Pica N, Palese P. Toward a universal influenza virus vaccine: prospects and challenges. Annu Rev Med. 2013; 64:189–202.
Article7. Shane HL, Klonowski KD. Every breath you take: the impact of environment on resident memory CD8 T cells in the lung. Front Immunol. 2014; 5:320.
Article8. Damjanovic D, Small CL, Jeyanathan M, McCormick S, Xing Z. Immunopathology in influenza virus infection: uncoupling the friend from foe. Clin Immunol. 2012; 144:57–69.
Article9. Kuiken T, Riteau B, Fouchier RA, Rimmelzwaan GF. Pathogenesis of influenza virus infections: the good, the bad and the ugly. Curr Opin Virol. 2012; 2:276–286.
Article10. Brandes M, Klauschen F, Kuchen S, Germain RN. A systems analysis identifies a feedforward inflammatory circuit leading to lethal influenza infection. Cell. 2013; 154:197–212.
Article11. Tate MD, Ioannidis LJ, Croker B, Brown LE, Brooks AG, Reading PC. The role of neutrophils during mild and severe influenza virus infections of mice. PLoS One. 2011; 6:e17618.
Article12. Ji H, Gu Q, Chen LL, Xu K, Ling X, Bao CJ, Tang FY, Qi X, Wu YQ, Ai J, et al. Epidemiological and clinical characteristics and risk factors for death of patients with avian influenza A H7N9 virus infection from Jiangsu Province, Eastern China. PLoS One. 2014; 9:e89581.
Article13. Maines TR, Szretter KJ, Perrone L, Belser JA, Bright RA, Zeng H, Tumpey TM, Katz JM. Pathogenesis of emerging avian influenza viruses in mammals and the host innate immune response. Immunol Rev. 2008; 225:68–84.
Article14. Wilkinson TM, Li CK, Chui CS, Huang AK, Perkins M, Liebner JC, Lambkin-Williams R, Gilbert A, Oxford J, Nicholas B, et al. Preexisting influenza-specific CD4+ T cells correlate with disease protection against influenza challenge in humans. Nat Med. 2012; 18:274–280.
Article15. Kang MC, Choi DH, Choi YW, Park SJ, Namkoong H, Park KS, Ahn SS, Surh CD, Yoon SW, Kim DJ, et al. Intranasal introduction of Fc-fused interleukin-7 provides long-lasting prophylaxis against lethal influenza virus infection. J Virol. 2015; 90:2273–2284.
Article16. Nam HJ, Song MY, Choi DH, Yang SH, Jin HT, Sung YC. Marked enhancement of antigen-specific T-cell responses by IL-7-fused nonlytic, but not lytic, Fc as a genetic adjuvant. Eur J Immunol. 2010; 40:351–358.
Article17. Mackall CL, Fry TJ, Gress RE. Harnessing the biology of IL-7 for therapeutic application. Nat Rev Immunol. 2011; 11:330–342.
Article18. Turner DL, Bickham KL, Thome JJ, Kim CY, D'Ovidio F, Wherry EJ, Farber DL. Lung niches for the generation and maintenance of tissue-resident memory T cells. Mucosal Immunol. 2014; 7:501–510.
Article19. Langlois RA, Legge KL. Plasmacytoid dendritic cells enhance mortality during lethal influenza infections by eliminating virus-specific CD8 T cells. J Immunol. 2010; 184:4440–4446.
Article20. Soloff AC, Weirback HK, Ross TM, Barratt-Boyes SM. Plasmacytoid dendritic cell depletion leads to an enhanced mononuclear phagocyte response in lungs of mice with lethal influenza virus infection. Comp Immunol Microbiol Infect Dis. 2012; 35:309–317.
Article21. Jewell NA, Vaghefi N, Mertz SE, Akter P, Peebles RS Jr, Bakaletz LO, Durbin RK, Flaño E, Durbin JE. Differential type I interferon induction by respiratory syncytial virus and influenza a virus in vivo. J Virol. 2007; 81:9790–9800.
Article22. Arimori Y, Nakamura R, Yamada H, Shibata K, Maeda N, Kase T, Yoshikai Y. Type I interferon limits influenza virus-induced acute lung injury by regulation of excessive inflammation in mice. Antiviral Res. 2013; 99:230–237.
Article23. Hong J, Gong ZJ. Human plasmacytoid dendritic cells from patients with chronic hepatitis B virus infection induce the generation of a higher proportion of CD4(+) and CD25(+) regulatory T cells compared with healthy patients. Hepatol Res. 2008; 38:362–373.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Erratum: Plasmacytoid Dendritic Cells Contribute to the Protective Immunity Induced by Intranasal Treatment with Fc-fused Interleukin-7 against Lethal Influenza Virus Infection
- Mucosal Immunization with Recombinant Adenovirus Encoding Soluble Globular Head of Hemagglutinin Protects Mice Against Lethal Influenza Virus Infection
- Vaccine Strategy That Enhances the Protective Efficacy of Systemic Immunization by Establishing LungResident Memory CD8 T Cells Against Influenza Infection
- Mechanisms of Cross-protection by Influenza Virus M2-based Vaccines
- Distinct Effects of Monophosphoryl Lipid A, Oligodeoxynucleotide CpG, and Combination Adjuvants on Modulating Innate and Adaptive Immune Responses to Influenza Vaccination