Asia Pac Allergy.
2016 Jul;6(3):168-173. 10.5415/apallergy.2016.6.3.168.
Effect on quality of life of the mixed house dust mite/weed pollen extract immunotherapy
- Affiliations
-
- 1Allergy Department, Peking Union Medical College Hospital, Beijing 100730, China. dr_guankai@126.com
- KMID: 2400418
- DOI: http://doi.org/10.5415/apallergy.2016.6.3.168
Abstract
- BACKGROUND
Although many patients with allergic rhinitis have symptoms due to sensitization to more than one kind of allergens, and mixed allergen extracts are widely used for immunotherapy, there are few published trials.
OBJECTIVE
Our study aimed to evaluate the effect of multiple-allergen immunotherapy on improving the symptoms and quality of life of allergic rhinitis patients.
METHODS
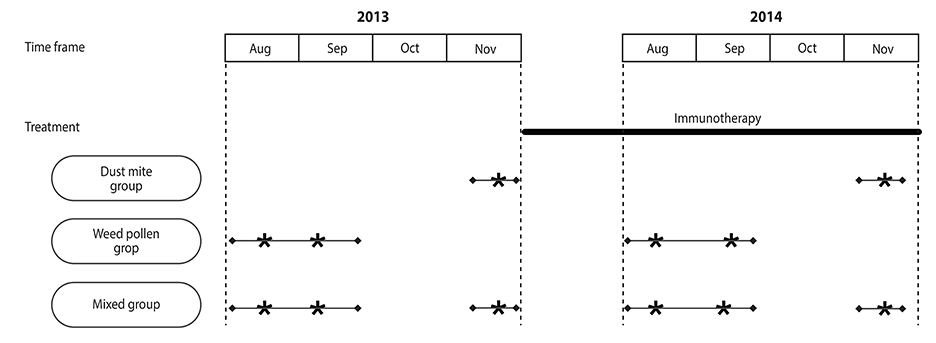
We performed a 1-year single-center observation study of subcutaneous immunotherapy using house dust mite extract (n = 12), weed pollen extract (n = 21), or mixed house dust mite/weed pollen extract (n = 11) in 44 allergic rhinitis patients. All the allergens responsible for the symptom of each patient were included in his immunotherapy. Symptom score, medication score, and quality of life of the patients were evaluated before and after 1-year immunotherapy. Quality of life was evaluated with the Rhinoconjunctivitis Quality of Life Questionnaire.
RESULTS
In all 3 groups receiving subcutaneous immunotherapy, significant improvement of symptom score, medication score, and quality of life was found vs. baseline at 1 year, irrespective of the allergen used. In the weed pollen season, the changes of quality of life questionnaire score after 1-year treatment were not significantly different between the weed pollen group (1.55 ± 1.24) and the mixed house dust mite/weed pollen group (1.14 ± 1.01). The same happened in the nonpollen seasons, during which dust mite immunotherapy (1.23 ± 1.63) and mixed immunotherapy (0.60 ± 0.47) did not show significantly different effect on the quality of life.
CONCLUSION
The multiple-allergen immunotherapy might be effective in polysensitized allergic rhinitis patients, and could improve their quality of life. Our result did not show significant difference between the effects of multiple-allergen immunotherapy and mono-allergen immunotherapy.
MeSH Terms
Figure
Cited by 3 articles
-
Advances in technology are changing the future of medicine
Yoon-Seok Chang
Asia Pac Allergy. 2016;6(3):137-138. doi: 10.5415/apallergy.2016.6.3.137.Changes in skin reactivity and associated factors in patients sensitized to house dust mites after 1 year of allergen-specific immunotherapy
Jeong-Yeop Son, Mann-Hong Jung, Kwang-Wook Koh, Eun-Kee Park, Jeong-Hoon Heo, Gil-Soon Choi, Hee-Kyoo Kim
Asia Pac Allergy. 2017;7(2):82-91. doi: 10.5415/apallergy.2017.7.2.82.Allergic conjunctivitis in Asia
Bernard Yu-Hor Thong
Asia Pac Allergy. 2017;7(2):57-64. doi: 10.5415/apallergy.2017.7.2.57.
Reference
-
1. Bousquet J, Lockey R, Malling HJ. Allergen immunotherapy: therapeutical vaccines for allergic diseases. A WHO position paper. J Allergy Clin Immunol. 1998; 102:558–562.2. Li Q, Li M, Yue W, Zhou J, Li R, Lin J, Li Y. Predictive factors for clinical response to allergy immunotherapy in children with asthma and rhinitis. Int Arch Allergy Immunol. 2014; 164:210–217.
Article3. Bousquet PJ, Castelli C, Daures JP, Heinrich J, Hooper R, Sunyer J, Wjst M, Jarvis D, Burney P. Assessment of allergen sensitization in a general population-based survey (European Community Respiratory Health Survey I). Ann Epidemiol. 2010; 20:797–803.
Article4. Juniper EF, Thompson AK, Ferrie PJ, Roberts JN. Validation of the standardized version of the Rhinoconjunctivitis Quality of Life Questionnaire. J Allergy Clin Immunol. 1999; 104(2 Pt 1):364–369.
Article5. Nelson HS. Multiallergen immunotherapy for allergic rhinitis and asthma. J Allergy Clin Immunol. 2009; 123:763–769.
Article6. Nelson HS, Iklé D, Buchmeier A. Studies of allergen extract stability: the effects of dilution and mixing. J Allergy Clin Immunol. 1996; 98:382–388.
Article7. Nelson HS. Specific immunotherapy with allergen mixes: what is the evidence? Curr Opin Allergy Clin Immunol. 2009; 9:549–553.
Article8. Esch RE. Allergen immunotherapy: what can and cannot be mixed? J Allergy Clin Immunol. 2008; 122:659–660.
Article9. Bousquet J, Becker WM, Hejjaoui A, Chanal I, Lebel B, Dhivert H, Michel FB. Differences in clinical and immunologic reactivity of patients allergic to grass pollens and to multiple-pollen species. II. Efficacy of a double-blind, placebo-controlled, specific immunotherapy with standardized extracts. J Allergy Clin Immunol. 1991; 88:43–53.10. Adkinson NF Jr, Eggleston PA, Eney D, Goldstein EO, Schuberth KC, Bacon JR, Hamilton RG, Weiss ME, Arshad H, Meinert CL, Tonascia J, Wheeler B. A controlled trial of immunotherapy for asthma in allergic children. N Engl J Med. 1997; 336:324–331.
Article11. Alvarez-Cuesta E, Aragoneses-Gilsanz E, Martin-Garcia C, Berges-Gimeno P, Gonzalez-Mancebo E, Cuesta-Herranz J. Immunotherapy with depigmented glutaraldehyde-polymerized extracts: changes in quality of life. Clin Exp Allergy. 2005; 35:572–578.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Change of causative inhalant allergens in respiratory allergic patients in Chungbuk district
- Effect of Asthma Management Educational Program on The Disease Related Knowledge, Stress, and Self-efficacy of Asthmatics Allergic to House Dust Mite
- Two Cases of Atopic Dermatitis Improved by Combination Treatment of Allergen-Specific Immunotherapy and Histamine-Immunoglobulin Complex
- Specific Antibody Response in House Dust Mite Asthmatics on Immunotherapy
- The effect of house dust mite conventional immunotherapy on the production of IL-4 and interferon-gamma from the peripheral blood T cells in asthmatic children