J Clin Neurol.
2009 Mar;5(1):1-10.
Bone Marrow-Derived Mesenchymal Stem Cell Therapy as a Candidate Disease-Modifying Strategy in Parkinson's Disease and Multiple System Atrophy
- Affiliations
-
- 1Department of Neurology, Yonsei University College of Medicine, Seoul, Korea. phlee@yuhs.ac
- 2Center for Neuroregeneration and Stem Cell Research, Ajou University College of Medicine, Suwon, Korea.
Abstract
- Parkinson's disease (PD) and multiple system atrophy (MSA) are neurodegenerative diseases representative of alpha-synucleinopathies characterized pathologically by alpha-synuclein-abundant Lewy bodies and glial cytoplasmic inclusions, respectively. Embryonic stem cells, fetal mesencephalic neurons, and neural stem cells have been introduced as restorative strategies in PD animals and patients, but ethical and immunological problems as well as the serious side effects of tumorigenesis and disabling dyskinesia have limited clinical application of these stem cells. Meanwhile, cell therapy using mesenchymal stem cells (MSCs) is attractive clinically because these cells are free from ethical and immunological problems. MSCs are present in adult bone marrow and represent <0.01% of all nucleated bone marrow cells. MSCs are themselves capable of multipotency, differentiating under appropriate conditions into chondrocytes, skeletal myocytes, and neurons. According to recent studies, the neuroprotective effect of MSCs is mediated by their ability to produce various trophic factors that contribute to functional recovery, neuronal cell survival, and stimulation of endogenous regeneration and by immunoregulatory properties that not only inhibit nearly all cells participating in the immune response cell-cell-contact-dependent mechanism, but also release various soluble factors associated with immunosuppressive activity. However, the use of MSCs as neuroprotectives in PD and MSA has seldom been studied. Here we comprehensively review recent advances in the therapeutic roles of MSCs in PD and MSA, especially focusing on their neuroprotective properties and use in disease-modifying therapeutic strategies.
Keyword
MeSH Terms
-
Adult
Animals
Bone Marrow
Bone Marrow Cells
Cell Survival
Cell Transformation, Neoplastic
Chondrocytes
Dyskinesias
Embryonic Stem Cells
Humans
Inclusion Bodies
Lewy Bodies
Mesenchymal Stromal Cells
Multiple System Atrophy
Muscle Fibers, Skeletal
Neural Stem Cells
Neurodegenerative Diseases
Neurons
Neuroprotective Agents
Parkinson Disease
Regeneration
Stem Cells
Tissue Therapy
Neuroprotective Agents
Figure
Reference
-
1. Moore DJ, West AB, Dawson VL, Dawson TM. Molecular pathophysiology of Parkinson's disease. Annu Rev Neurosci. 2005. 28:57–87.
Article2. von Bohlen und Halbach O, Schober A, Krieglstein K. Genes, proteins, and neurotoxins involved in Parkinson's disease. Prog Neurobiol. 2004. 73:151–177.
Article3. Adler CH. Nonmotor complications in Parkinson's disease. Mov Disord. 2005. 20:Suppl 11. S23–S29.
Article4. Palmer MR, Granholm AC, van Horne CG, Giardina KE, Freund RK, Moorhead JW, et al. Intranigral transplantation of solid tissue ventral mesencephalon or striatal grafts induces behavioral recovery in 6-OHDA-lesioned rats. Brain Res. 2001. 890:86–99.
Article5. Lindvall O. Stem cells for cell therapy in Parkinson's disease. Pharmacol Res. 2003. 47:279–287.
Article6. Freed CR, Greene PE, Breeze RE, Tsai WY, DuMouchel W, Kao R, et al. Transplantation of embryonic dopamine neurons for severe Parkinson's disease. N Engl J Med. 2001. 344:710–719.
Article7. Olanow CW, Goetz CG, Kordower JH, Stoessl AJ, Sossi V, Brin MF, et al. A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson's disease. Ann Neurol. 2003. 54:403–414.
Article8. Poewe W. Non-motor symptoms in Parkinson's disease. Eur J Neurol. 2008. 15 Suppl 1:14–20.
Article9. Braak H, Del Tredici K. Invited Article: Nervous system pathology in sporadic Parkinson disease. Neurology. 2008. 70:1916–1925.
Article10. Braak H, Ghebremedhin E, Rüb U, Bratzke H, Del Tredici K. Stages in the development of Parkinson's disease-related pathology. Cell Tissue Res. 2004. 318:121–134.
Article11. Lee PH, Yeo SH, Kim HJ, Youm HY. Correlation between cardiac 123I-MIBG and odor identification in patients with Parkinson's disease and multiple system atrophy. Mov Disord. 2006. 21:1975–1977.
Article12. Correia AS, Anisimov SV, Li JY, Brundin P. Stem cell-based therapy for Parkinson's disease. Ann Med. 2005. 37:487–498.
Article13. Hagell P, Piccini P, Björklund A, Brundin P, Rehncrona S, Widner H, et al. Dyskinesias following neural transplantation in Parkinson's disease. Nat Neurosci. 2002. 5:627–628.
Article14. Björklund A, Dunnett SB, Brundin P, Stoessl AJ, Freed CR, Breeze RE, et al. Neural transplantation for the treatment of Parkinson's disease. Lancet Neurol. 2003. 2:437–445.
Article15. Winkler C, Kirik D, Björklund A. Cell transplantation in Parkinson's disease: how can we make it work? Trends Neurosci. 2005. 28:86–92.
Article16. Isacson O, Bjorklund LM, Schumacher JM. Toward full restoration of synaptic and terminal function of the dopaminergic system in Parkinson's disease by stem cells. Ann Neurol. 2003. 53:Suppl 3. S135–S146. discussion S146-S148.
Article17. Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson's disease. Nat Med. 2008. 14:504–506.
Article18. Li JY, Englund E, Holton JL, Soulet D, Hagell P, Lees AJ, et al. Lewy bodies in grafted neurons in subjects with Parkinson's disease suggest host-to-graft disease propagation. Nat Med. 2008. 14:501–503.
Article19. Mendez I, Viñuela A, Astradsson A, Mukhida K, Hallett P, Robertson H, et al. Dopamine neurons implanted into people with Parkinson's disease survive without pathology for 14 years. Nat Med. 2008. 14:507–509.
Article20. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999. 284:143–147.
Article21. Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000. 61:364–370.
Article22. Minguell JJ, Erices A, Conget P. Mesenchymal stem cells. Exp Biol Med (Maywood). 2001. 226:507–520.
Article23. Bonuccelli U, Del Dotto P. New pharmacologic horizons in the treatment of Parkinson disease. Neurology. 2006. 67:S30–S38.
Article24. Li Y, Chen J, Chen XG, Wang L, Gautam SC, Xu YX, et al. Human marrow stromal cell therapy for stroke in rat: neurotrophins and functional recovery. Neurology. 2002. 59:514–523.
Article25. Mahmood A, Lu D, Chopp M. Marrow stromal cell transplantation after traumatic brain injury promotes cellular proliferation within the brain. Neurosurgery. 2004. 55:1185–1193.
Article26. Crigler L, Robey RC, Asawachaicharn A, Gaupp D, Phinney DG. Human mesenchymal stem cell subpopulations express a variety of neuro-regulatory molecules and promote neuronal cell survival and neuritogenesis. Exp Neurol. 2006. 198:54–64.
Article27. Arnhold S, Klein H, Klinz FJ, Absenger Y, Schmidt A, Schinköthe T, et al. Human bone marrow stroma cells display certain neural characteristics and integrate in the subventricular compartment after injection into the liquor system. Eur J Cell Biol. 2006. 85:551–565.
Article28. Barry FP, Murphy JM. Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol. 2004. 36:568–584.
Article29. McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson's and Alzheimer's disease brains. Neurology. 1988. 38:1285–1291.
Article30. Langston JW, Forno LS, Tetrud J, Reeves AG, Kaplan JA, Karluk D. Evidence of active nerve cell degeneration in the substantia nigra of humans years after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine exposure. Ann Neurol. 1999. 46:598–605.
Article31. Ouchi Y, Yoshikawa E, Sekine Y, Futatsubashi M, Kanno T, Ogusu T, et al. Microglial activation and dopamine terminal loss in early Parkinson's disease. Ann Neurol. 2005. 57:168–175.
Article32. Hunot S, Dugas N, Faucheux B, Hartmann A, Tardieu M, Debré P, et al. FcepsilonRII/CD23 is expressed in Parkinson's disease and induces, in vitro, production of nitric oxide and tumor necrosis factor-alpha in glial cells. J Neurosci. 1999. 19:3440–3447.
Article33. Nagatsu T, Mogi M, Ichinose H, Togari A. Cytokines in Parkinson's disease. J Neural Transm Suppl. 2000. 143–151.
Article34. Gao HM, Hong JS, Zhang W, Liu B. Distinct role for microglia in rotenone-induced degeneration of dopaminergic neurons. J Neurosci. 2002. 22:782–790.
Article35. Cicchetti F, Brownell AL, Williams K, Chen YI, Livni E, Isacson O. Neuroinflammation of the nigrostriatal pathway during progressive 6-OHDA dopamine degeneration in rats monitored by immunohistochemistry and PET imaging. Eur J Neurosci. 2002. 15:991–998.
Article36. Liberatore GT, Jackson-Lewis V, Vukosavic S, Mandir AS, Vila M, McAuliffe WG, et al. Inducible nitric oxide synthase stimulates dopaminergic neurodegeneration in the MPTP model of Parkinson disease. Nat Med. 1999. 5:1403–1409.
Article37. Krampera M, Pasini A, Pizzolo G, Cosmi L, Romagnani S, Annunziato F. Regenerative and immunomodulatory potential of mesenchymal stem cells. Curr Opin Pharmacol. 2006. 6:435–441.
Article38. Karussis D, Kassis I, Kurkalli BG, Slavin S. Immunomodulation and neuroprotection with mesenchymal bone marrow stem cells (MSCs): a proposed treatment for multiple sclerosis and other neuroimmunological/neurodegenerative diseases. J Neurol Sci. 2008. 265:131–135.
Article39. Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007. 110:3499–3506.
Article40. Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E, et al. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005. 106:1755–1761.
Article41. Gerdoni E, Gallo B, Casazza S, Musio S, Bonanni I, Pedemonte E, et al. Mesenchymal stem cells effectively modulate pathogenic immune response in experimental autoimmune encephalomyelitis. Ann Neurol. 2007. 61:219–227.
Article42. Guo J, Lin GS, Bao CY, Hu ZM, Hu MY. Anti-inflammation role for mesenchymal stem cells transplantation in myocardial infarction. Inflammation. 2007. 30:97–104.
Article43. Aubin N, Curet O, Deffois A, Carter C. Aspirin and salicylate protect against MPTP-induced dopamine depletion in mice. J Neurochem. 1998. 71:1635–1642.
Article44. Teismann P, Tieu K, Choi DK, Wu DC, Naini A, Hunot S, et al. Cyclooxygenase-2 is instrumental in Parkinson's disease neurodegeneration. Proc Natl Acad Sci U S A. 2003. 100:5473–5478.
Article45. Chen H, Zhang SM, Hernán MA, Schwarzschild MA, Willett WC, Colditz GA, et al. Nonsteroidal anti-inflammatory drugs and the risk of Parkinson disease. Arch Neurol. 2003. 60:1059–1064.
Article46. Wahner AD, Bronstein JM, Bordelon YM, Ritz B. Nonsteroidal anti-inflammatory drugs may protect against Parkinson disease. Neurology. 2007. 69:1836–1842.
Article47. Chen J, Li Y, Katakowski M, Chen X, Wang L, Lu D, et al. Intravenous bone marrow stromal cell therapy reduces apoptosis and promotes endogenous cell proliferation after stroke in female rat. J Neurosci Res. 2003. 73:778–786.
Article48. Li Y, Chen J, Wang L, Zhang L, Lu M, Chopp M. Intracerebral transplantation of bone marrow stromal cells in a 1-methyl-4-phenyl-1,2,3, 6-tetrahydropyridine mouse model of Parkinson's disease. Neurosci Lett. 2001. 316:67–70.
Article49. Stumm RK, Rummel J, Junker V, Culmsee C, Pfeiffer M, Krieglstein J, et al. A dual role for the SDF-1/CXCR4 chemokine receptor system in adult brain: isoform-selective regulation of SDF-1 expression modulates CXCR4-dependent neuronal plasticity and cerebral leukocyte recruitment after focal ischemia. J Neurosci. 2002. 22:5865–5878.
Article50. Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007. 25:2739–2749.
Article51. Banisadr G, Skrzydelski D, Kitabgi P, Rosténe W, Parsadaniantz SM. Highly regionalized distribution of stromal cell-derived factor-1/CXCL12 in adult rat brain: constitutive expression in cholinergic, dopaminergic and vasopressinergic neurons. Eur J Neurosci. 2003. 18:1593–1606.
Article52. Emborg ME. Evaluation of animal models of Parkinson's disease for neuroprotective strategies. J Neurosci Methods. 2004. 139:121–143.
Article53. McNaught KS, Perl DP, Brownell AL, Olanow CW. Systemic exposure to proteasome inhibitors causes a progressive model of Parkinson's disease. Ann Neurol. 2004. 56:149–162.
Article54. Schapira AH, Cleeter MW, Muddle JR, Workman JM, Cooper JM, King RH. Proteasomal inhibition causes loss of nigral tyrosine hydroxylase neurons. Ann Neurol. 2006. 60:253–255.
Article55. Zeng BY, Bukhatwa S, Hikima A, Rose S, Jenner P. Reproducible nigral cell loss after systemic proteasomal inhibitor administration to rats. Ann Neurol. 2006. 60:248–252.
Article56. Bové J, Zhou C, Jackson-Lewis V, Taylor J, Chu Y, Rideout HJ, et al. Proteasome inhibition and Parkinson's disease modeling. Ann Neurol. 2006. 60:260–264.
Article57. Kordower JH, Kanaan NM, Chu Y, Suresh Babu R, Stansell J 3rd, Terpstra BT, et al. Failure of proteasome inhibitor administration to provide a model of Parkinson's disease in rats and monkeys. Ann Neurol. 2006. 60:264–268.
Article58. Manning-Boğ AB, Reaney SH, Chou VP, Johnston LC, McCormack AL, Johnston J, et al. Lack of nigrostriatal pathology in a rat model of proteasome inhibition. Ann Neurol. 2006. 60:256–260.
Article59. Park HJ, Lee PH, Bang OY, Lee G, Ahn YH. Mesenchymal stem cells therapy exerts neuroprotection in a progressive animal model of Parkinson's disease. J Neurochem. 2008. 107:141–151.
Article60. Tatton WG, Chalmers-Redman R, Brown D, Tatton N. Apoptosis in Parkinson's disease: signals for neuronal degradation. Ann Neurol. 2003. 53:Suppl 3. S61–S70. discussion S70-S72.
Article61. Olanow CW, McNaught KS. Ubiquitin-proteasome system and Parkinson's disease. Mov Disord. 2006. 21:1806–1823.
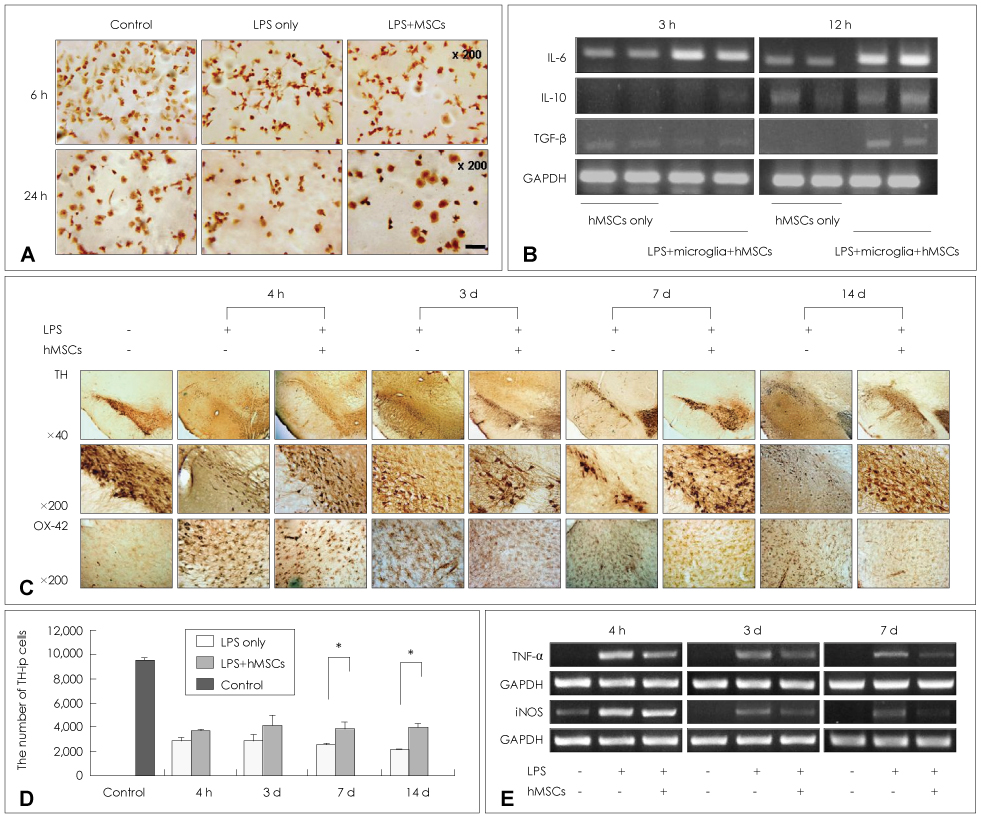
Article62. Kim YJ, Park HJ, Lee G, Bang OY, Ahn YH, Joe E, et al. Neuroprotective effects of human mesenchymal stem cells on dopaminergic neurons through anti-inflammatory action. Glia. 2009. 57:13–23.
Article63. Mareschi K, Novara M, Rustichelli D, Ferrero I, Guido D, Carbone E, et al. Neural differentiation of human mesenchymal stem cells: Evidence for expression of neural markers and eag K+ channel types. Exp Hematol. 2006. 34:1563–1572.
Article64. Blondheim NR, Levy YS, Ben-Zur T, Burshtein A, Cherlow T, Kan I, et al. Human mesenchymal stem cells express neural genes, suggesting a neural predisposition. Stem Cells Dev. 2006. 15:141–164.
Article65. Barzilay R, Kan I, Ben-Zur T, Bulvik S, Melamed E, Offen D. Induction of human mesenchymal stem cells into dopamine-producing cells with different differentiation protocols. Stem Cells Dev. 2008. 17:547–554.
Article66. Offen D, Barhum Y, Levy YS, Burshtein A, Panet H, Cherlow T, et al. Intrastriatal transplantation of mouse bone marrow-derived stem cells improves motor behavior in a mouse model of Parkinson's disease. J Neural Transm Suppl. 2007. 133–143.
Article67. Ye M, Wang XJ, Zhang YH, Lu GQ, Liang L, Xu JY, et al. Therapeutic effects of differentiated bone marrow stromal cell transplantation on rat models of Parkinson's disease. Parkinsonism Relat Disord. 2007. 13:44–49.
Article68. Wenning GK, Colosimo C, Geser F, Poewe W. Multiple system atrophy. Lancet Neurol. 2004. 3:93–103.
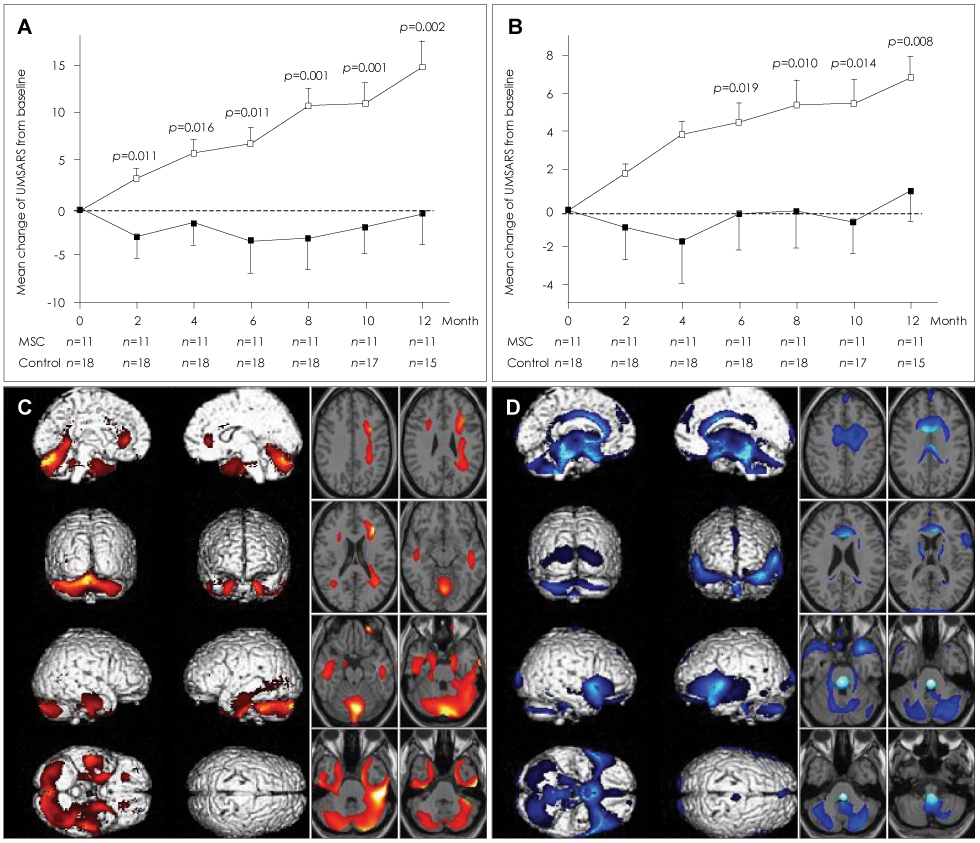
Article69. Lee PH, Kim JW, Bang OY, Ahn YH, Joo IS, Huh K. Autologous mesenchymal stem cell therapy delays the progression of neurological deficits in patients with multiple system atrophy. Clin Pharmacol Ther. 2008. 83:723–730.
Article70. Quinn N, Barker RA, Wenning GK. Are trials of intravascular infusions of autologous mesenchymal stem cells in patients with multiple system atrophy currently justified, and are they effective? Clin Pharmacol Ther. 2008. 83:663–665.
Article71. Whone AL, Scolding NJ. Mesenchymal stem cells and neurodegenerative disease. Clin Pharmacol Ther. 2009. 85:19–20.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Treatment Strategy for Parkinsonian Diseases Through Mesenchymal Stem Cells
- Clinical Safety and Efficacy of Autologous Bone Marrow-Derived Mesenchymal Stem Cell Transplantation in Sensorineural Hearing Loss Patients
- Concise Review: Differentiation of Human Adult Stem Cells Into Hepatocyte-like Cells In vitro
- Therapeutic Aspects of Mesenchymal Stem Cell-Based Cell Therapy with a Focus on Human Amniotic Epithelial Cells in Multiple Sclerosis: A Mechanistic Review
- Stem Cells and Niemann Pick Disease