Ann Dermatol.
2018 Feb;30(1):47-53. 10.5021/ad.2018.30.1.47.
Connective Tissue Growth Factor Neutralization Aggravates the Psoriasis Skin Lesion: The Analysis of Psoriasis Model Mice and Patients
- Affiliations
-
- 1Institutes for Environmental and Gender Specific Medicine, Juntendo University Graduate School of Medicine, Chiba, Japan. keigo@juntendo.ac.jp
- 2Department of Internal Medicine and Rheumatology, Juntendo University Urayasu Hospital, Chiba, Japan.
- 3Department of Dermatology, Juntendo University Urayasu Hospital, Chiba, Japan.
- KMID: 2399754
- DOI: http://doi.org/10.5021/ad.2018.30.1.47
Abstract
- BACKGROUND
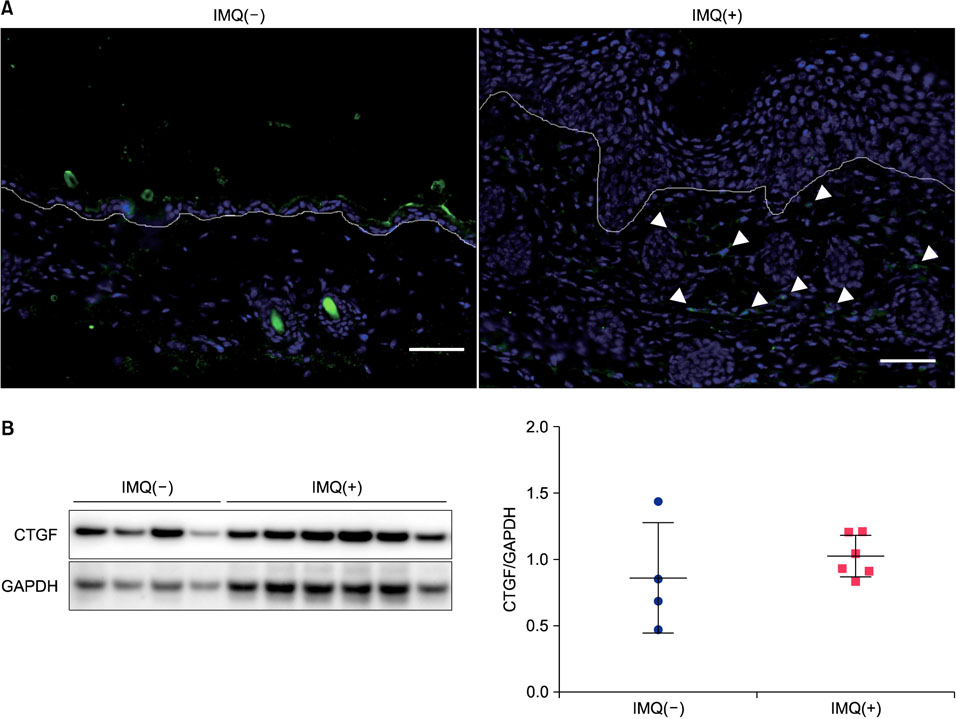
Connective tissue growth factor (CTGF) is a multifunctional cellular protein and playing a role as a central mediator in tissue remodeling and fibrosis. The physiological function of CTGF in psoriasis is unknown.
OBJECTIVE
The purpose of this study was to investigate the function of CTGF in psoriasis using the established imiquimod (IMQ)-induced psoriasis murine model and psoriasis patients.
METHODS
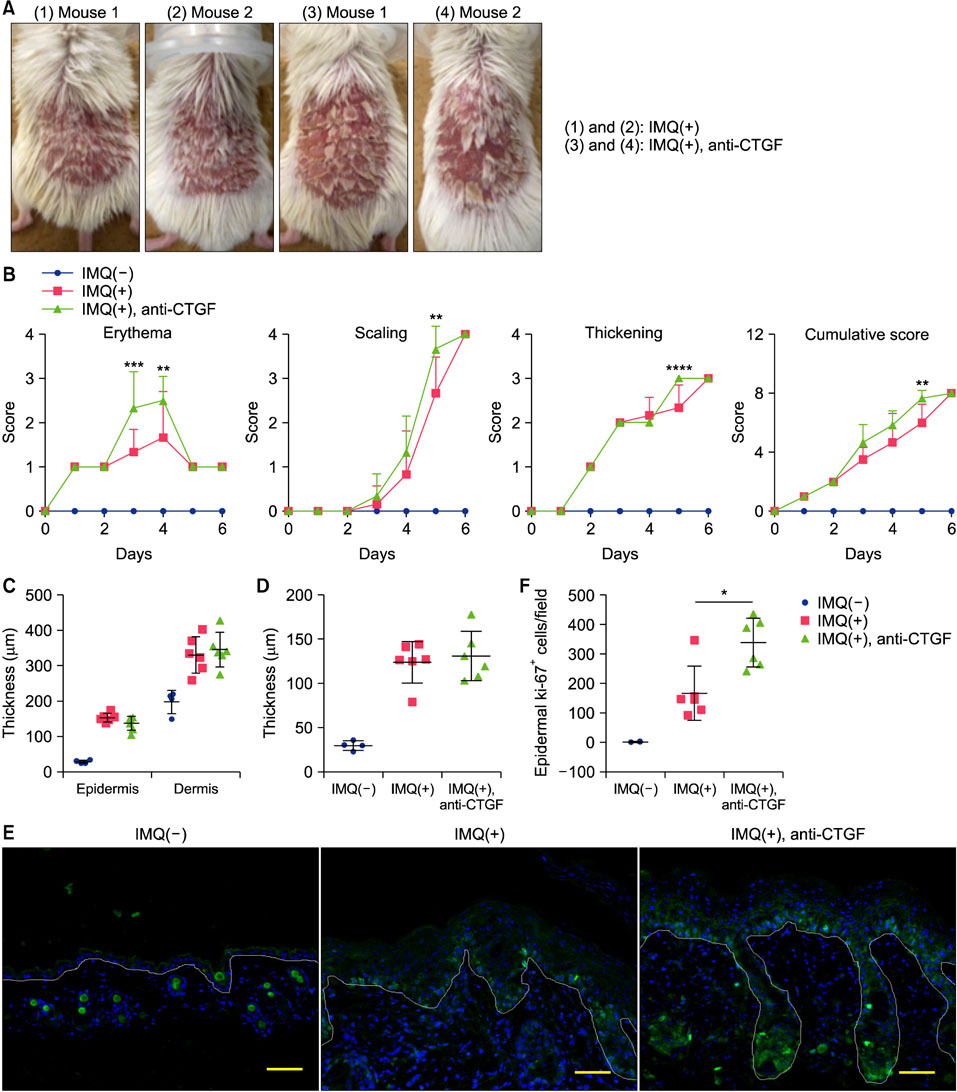
Anti-CTGF monoclonal antibody was applied to IMQ induced psoriasis mice and those skin were clinically, pathologically and immunologically analyzed. Additionally, CTGF expression was analyzes using skin samples and plasma from psoriasis patients.
RESULTS
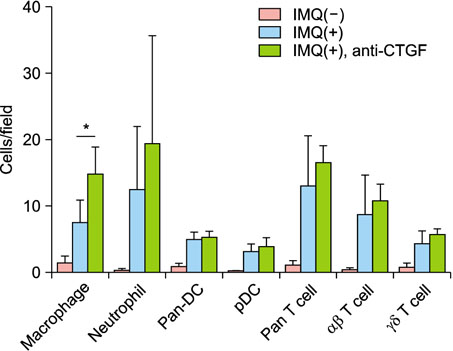
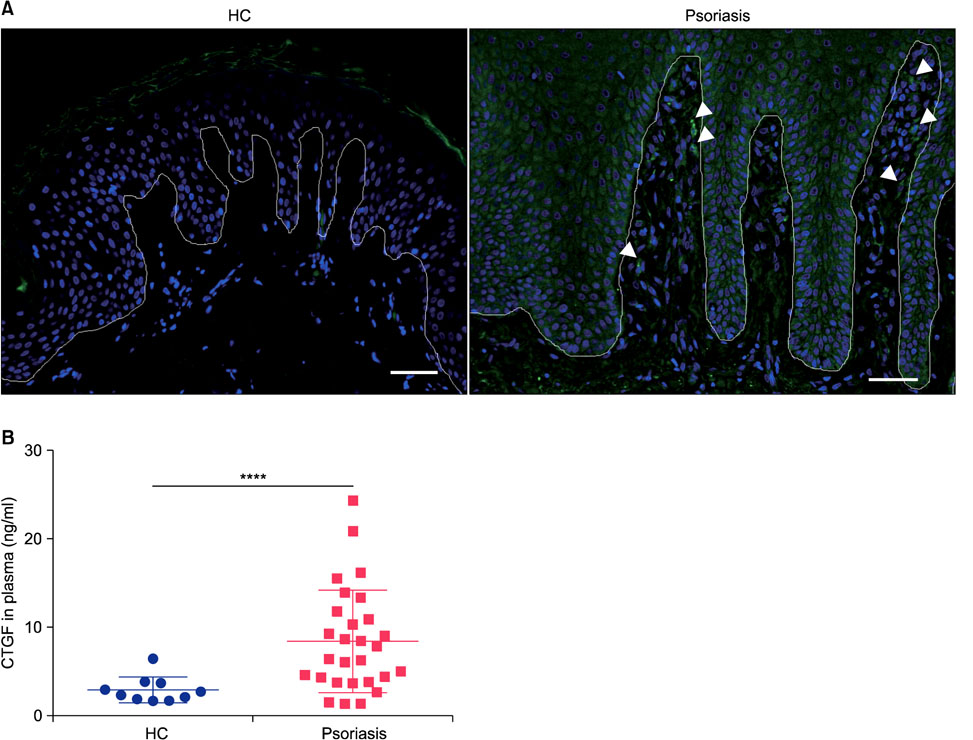
CTGF expression was observed in the dermis from both IMQ-induced psoriatic mice and psoriasis patients. CTGF inhibition using an anti-CTGF antibody slightly worsened IMQ-induced dermatitis. In addition, the increase of CTGF showed tendency to suppress the psoriatic dermatitis through inhibition of suprabasal cells proliferation and macrophage infiltration in the skin. CTGF was also detected significantly higher in plasma from psoriasis patients comparing with healthy control.
CONCLUSION
Our findings suggest that CTGF could contribute to the healing rather than the worsening of psoriasis skin lesions.
MeSH Terms
Figure
Reference
-
1. Leask A, Denton CP, Abraham DJ. Insights into the molecular mechanism of chronic fibrosis: the role of connective tissue growth factor in scleroderma. J Invest Dermatol. 2004; 122:1–6.
Article2. Igarashi A, Nashiro K, Kikuchi K, Sato S, Ihn H, Grotendorst GR, et al. Significant correlation between connective tissue growth factor gene expression and skin sclerosis in tissue sections from patients with systemic sclerosis. J Invest Dermatol. 1995; 105:280–284.
Article3. Takigawa M, Nakanishi T, Kubota S, Nishida T. Role of CTGF/HCS24/ecogenin in skeletal growth control. J Cell Physiol. 2003; 194:256–266.
Article4. Nozawa K, Fujishiro M, Kawasaki M, Kaneko H, Iwabuchi K, Yanagida M, et al. Connective tissue growth factor promotes articular damage by increased osteoclastogenesis in patients with rheumatoid arthritis. Arthritis Res Ther. 2009; 11:R174.
Article5. Nozawa K, Fujishiro M, Kawasaki M, Yamaguchi A, Ikeda K, Morimoto S, et al. Inhibition of connective tissue growth factor ameliorates disease in a murine model of rheumatoid arthritis. Arthritis Rheum. 2013; 65:1477–1486.
Article6. Lowes MA, Suárez-Fariñas M, Krueger JG. Immunology of psoriasis. Annu Rev Immunol. 2014; 32:227–255.
Article7. Reich K, Nestle FO, Papp K, Ortonne JP, Evans R, Guzzo C, et al. Infliximab induction and maintenance therapy for moderate-to-severe psoriasis: a phase III, multicentre, double-blind trial. Lancet. 2005; 366:1367–1374.
Article8. Coates LC, FitzGerald O, Helliwell PS, Paul C. Psoriasis, psoriatic arthritis, and rheumatoid arthritis: is all inflammation the same? Semin Arthritis Rheum. 2016; 46:291–304.
Article9. van der Fits L, Mourits S, Voerman JS, Kant M, Boon L, Laman JD, et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J Immunol. 2009; 182:5836–5845.
Article10. Miyazaki O, Kurashita S, Fukamachi I, Endo K, Ng PS, Takehara K. Subtraction method for determination of N-terminal connective tissue growth factor. Ann Clin Biochem. 2010; 47:205–211.
Article11. Fujishiro M, Yamaguchi A, Kawasaki M, Nozawa K, Takasaki Y, Takamori K, et al. The detection of plasma levels of connective tissue growth factor in rheumatoid arthritis patients. Clin Exp Rheumatol. 2012; 30:145–146.12. Swindell WR, Johnston A, Carbajal S, Han G, Wohn C, Lu J, et al. Genome-wide expression profiling of five mouse models identifies similarities and differences with human psoriasis. PLoS One. 2011; 6:e18266.
Article13. DiPietro LA. Wound healing: the role of the macrophage and other immune cells. Shock. 1995; 4:233–240.14. Waugh HV, Sherratt JA. Macrophage dynamics in diabetic wound dealing. Bull Math Biol. 2006; 68:197–207.
Article15. Alfaro MP, Deskins DL, Wallus M, DasGupta J, Davidson JM, Nanney LB, et al. A physiological role for connective tissue growth factor in early wound healing. Lab Invest. 2013; 93:81–95.
Article16. Henshaw FR, Boughton P, Lo L, McLennan SV, Twigg SM. Topically applied connective tissue growth factor/CCN2 improves diabetic preclinical cutaneous wound healing: potential role for CTGF in human diabetic foot ulcer healing. J Diabetes Res. 2015; 2015:236238.
Article17. Riley KG, Pasek RC, Maulis MF, Dunn JC, Bolus WR, Kendall PL, et al. Macrophages are essential for CTGF-mediated adult β-cell proliferation after injury. Mol Metab. 2015; 4:584–591.
Article18. Cicha I, Yilmaz A, Klein M, Raithel D, Brigstock DR, Daniel WG, et al. Connective tissue growth factor is overexpressed in complicated atherosclerotic plaques and induces mononuclear cell chemotaxis in vitro. Arterioscler Thromb Vasc Biol. 2005; 25:1008–1013.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Infliximab: Effective Therapy for Pustular Psoriasis
- A Case of Psoriasis Strictly Localized on a Vitiligo Lesion
- Increased expression of the epidermal growth factor receptor gene in psoriasis
- Clinical Study on Psoriasis - 2 . Classification of Severity and Comparative Study by the Activity of Psoriasis
- Recurrent Koebner Phenomenon in Psoriasis after Skin Grafts