J Periodontal Implant Sci.
2017 Dec;47(6):381-387. 10.5051/jpis.2017.47.6.381.
Ridge preservation using basic fibroblast growth factor-2 and collagenated biphasic calcium phosphate in beagle dogs
- Affiliations
-
- 1Department of Periodontology, Dental Research Institute, Seoul National University School of Dentistry, Seoul, Korea. kst72@snu.ac.kr
- 2Department of Restorative Dentistry, University at Buffalo School of Dental Medicine, Buffalo, NY, USA.
- KMID: 2399744
- DOI: http://doi.org/10.5051/jpis.2017.47.6.381
Abstract
- PURPOSE
The aim of this study was to evaluate volumetric and histologic changes in edentulous alveolar ridge areas after ridge preservation using basic fibroblast growth factor-2 (bFGF-2) in combination with collagenated biphasic calcium phosphate (BCP).
METHODS
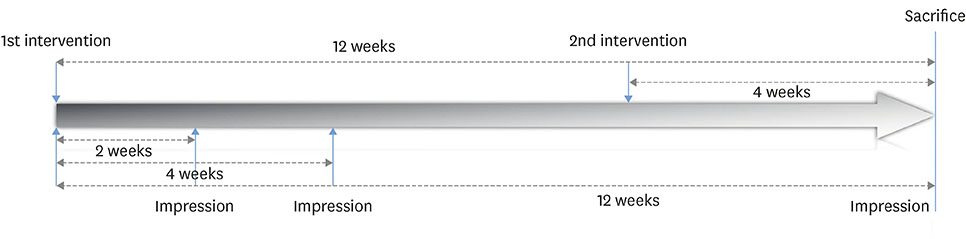
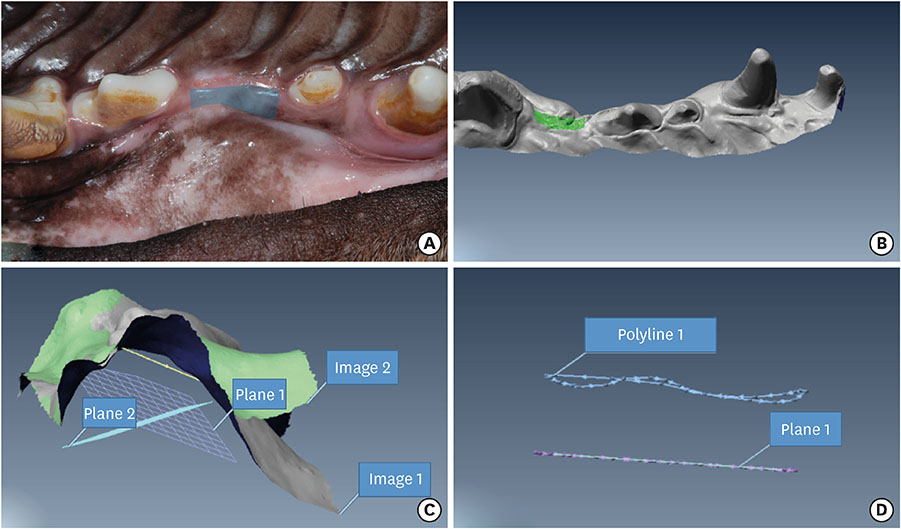
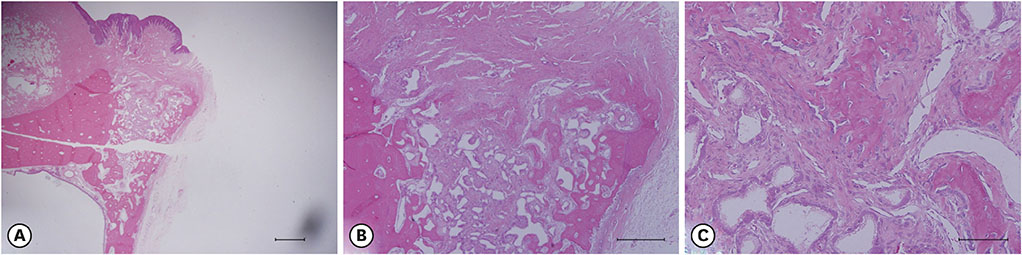
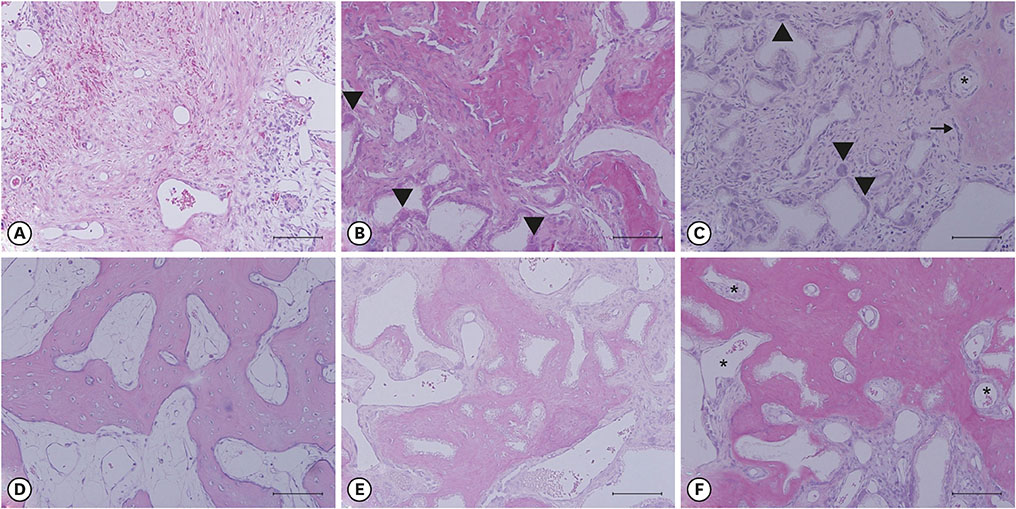
The experiments were performed in 6 adult male beagle dogs. The following 3 groups were created: 1) ridge preservation with bFGF-2 and collagenated BCP (experimental group), 2) ridge preservation with collagenated BCP (positive control group), and 3) a negative control group in which no ridge preservation procedure was performed. Volumetric change analysis was performed using an optical scanner and casts. Histological observations were made using light microscopy.
RESULTS
After the initial swelling subsided, the magnitude of the volumetric change in the experimental group and positive control group was smaller than in the negative control group. In the experimental group, a distinct trend was observed for the resorption of residual bone and collagen fibers at 4 weeks and for more mature bone and faster healing at 12 weeks.
CONCLUSIONS
Based on the findings of the present study, bFGF-2 may be considered for use as a therapeutic molecule in ridge preservation procedures.
Keyword
MeSH Terms
Figure
Reference
-
1. Pietrokovski J, Massler M. Alveolar ridge resorption following tooth extraction. J Prosthet Dent. 1967; 17:21–27.
Article2. Lam RV. Contour changes of the alveolar processes following extractions. J Prosthet Dent. 1960; 10:25–32.
Article3. Araújo MG, Lindhe J. Dimensional ridge alterations following tooth extraction. An experimental study in the dog. J Clin Periodontol. 2005; 32:212–218.
Article4. Schropp L, Wenzel A, Kostopoulos L, Karring T. Bone healing and soft tissue contour changes following single-tooth extraction: a clinical and radiographic 12-month prospective study. Int J Periodontics Restorative Dent. 2003; 23:313–323.5. Zubillaga G, Von Hagen S, Simon BI, Deasy MJ. Changes in alveolar bone height and width following post-extraction ridge augmentation using a fixed bioabsorbable membrane and demineralized freeze-dried bone osteoinductive graft. J Periodontol. 2003; 74:965–975.
Article6. Winkler S. Implant site development and alveolar bone resorption patterns. J Oral Implantol. 2002; 28:226–229.
Article7. Vignoletti F, Matesanz P, Rodrigo D, Figuero E, Martin C, Sanz M. Surgical protocols for ridge preservation after tooth extraction. A systematic review. Clin Oral Implants Res. 2012; 23:Suppl 5. 22–38.
Article8. Jung RE, Philipp A, Annen BM, Signorelli L, Thoma DS, Hämmerle CH, et al. Radiographic evaluation of different techniques for ridge preservation after tooth extraction: a randomized controlled clinical trial. J Clin Periodontol. 2013; 40:90–98.
Article9. Schneider D, Schmidlin PR, Philipp A, Annen BM, Ronay V, Hämmerle CH, et al. Labial soft tissue volume evaluation of different techniques for ridge preservation after tooth extraction: a randomized controlled clinical trial. J Clin Periodontol. 2014; 41:612–617.
Article10. Sato Y, Kikuchi M, Ohata N, Tamura M, Kuboki Y. Enhanced cementum formation in experimentally induced cementum defects of the root surface with the application of recombinant basic fibroblast growth factor in collagen gel in vivo . J Periodontol. 2004; 75:243–248.
Article11. Akagawa Y, Kubo T, Koretake K, Hayashi K, Doi K, Matsuura A, et al. Initial bone regeneration around fenestrated implants in Beagle dogs using basic fibroblast growth factor-gelatin hydrogel complex with varying biodegradation rates. J Prosthodont Res. 2009; 53:41–47.
Article12. Qu D, Li J, Li Y, Gao Y, Zuo Y, Hsu Y, et al. Angiogenesis and osteogenesis enhanced by bFGF ex vivo gene therapy for bone tissue engineering in reconstruction of calvarial defects. J Biomed Mater Res A. 2011; 96:543–551.13. Kim JS, Cha JK, Cho AR, Kim MS, Lee JS, Hong JY, et al. Acceleration of bone regeneration by BMP-2-loaded collagenated biphasic calcium phosphate in rabbit sinus. Clin Implant Dent Relat Res. 2015; 17:1103–1113.
Article14. Jung RE, Weber FE, Thoma DS, Ehrbar M, Cochran DL, Hämmerle CH. Bone morphogenetic protein-2 enhances bone formation when delivered by a synthetic matrix containing hydroxyapatite/tricalciumphosphate. Clin Oral Implants Res. 2008; 19:188–195.
Article15. Lu SX, Fiorini T, Lee J, Prasad HS, Buxton AN, Bisch FC, et al. Evaluation of a compression resistant matrix for recombinant human bone morphogenetic protein-2. J Clin Periodontol. 2013; 40:688–697.
Article16. Tsai CH, Chou MY, Jonas M, Tien YT, Chi EY. A composite graft material containing bone particles and collagen in osteoinduction in mouse. J Biomed Mater Res. 2002; 63:65–70.
Article17. Kodama N, Nagata M, Tabata Y, Ozeki M, Ninomiya T, Takagi R. A local bone anabolic effect of rhFGF2-impregnated gelatin hydrogel by promoting cell proliferation and coordinating osteoblastic differentiation. Bone. 2009; 44:699–707.
Article18. Fang TD, Salim A, Xia W, Nacamuli RP, Guccione S, Song HM, et al. Angiogenesis is required for successful bone induction during distraction osteogenesis. J Bone Miner Res. 2005; 20:1114–1124.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Histometrical evaluation of biphasic calcium phosphate in surgically created 1-wall periodontal intrabony defects in dogs
- Investigation of bone formation using calcium phosphate glass cement in beagle dogs
- The Effect of Recombinant Human Bone Morphogenetic Protein-2/Macroporous Biphasic Calcium Phosphate Block system on Bone Formation in Rat Calvarial Defects
- Histologic Study on the Effect of Two Types of Bovine Bone Powder in Extraction Socket of Beagle Dogs
- Effects of biphasic calcium phosphate on bone formation in human fetal osteoblasts