J Clin Neurol.
2018 Jan;14(1):35-42. 10.3988/jcn.2018.14.1.35.
Changes in the Common Carotid Artery after Radiotherapy: Wall Thickness, Calcification, and Atherosclerosis
- Affiliations
-
- 1Department of Neurology, Kyung Hee University Hospital, Kyung Hee University School of Medicine, Seoul, Korea.
- 2Department of Neurology, Chosun University Hospital, Gwangju, Korea.
- 3Department of Radiation Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 4Department of Neurology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. sukwon@amc.seoul.kr
- KMID: 2399597
- DOI: http://doi.org/10.3988/jcn.2018.14.1.35
Abstract
- BACKGROUND AND PURPOSE
Since the long-term survival rate has improved in laryngeal cancer patients who receive radiotherapy, concerns about postradiation complications (including carotid atherosclerosis) have increased. We followed changes in the common carotid artery (CCA) after radiotherapy and identified the underlying risk factors.
METHODS
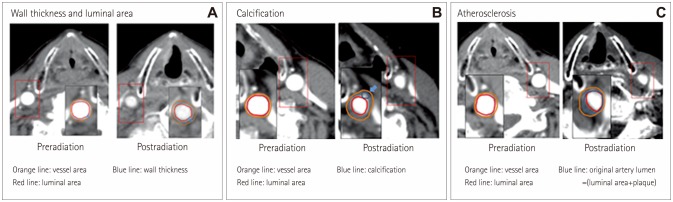
Consecutive patients with laryngeal cancer who underwent radiotherapy between January 1999 and December 2009 and who had received computed tomography (CT) both pre- and postradiotherapy were enrolled. Changes in the wall thickness and in the vessel and lumen areas as well as the presence of calcification or atherosclerosis were investigated. Demographics and risk factors were compared between patients with and without atherosclerosis at follow-up CT.
RESULTS
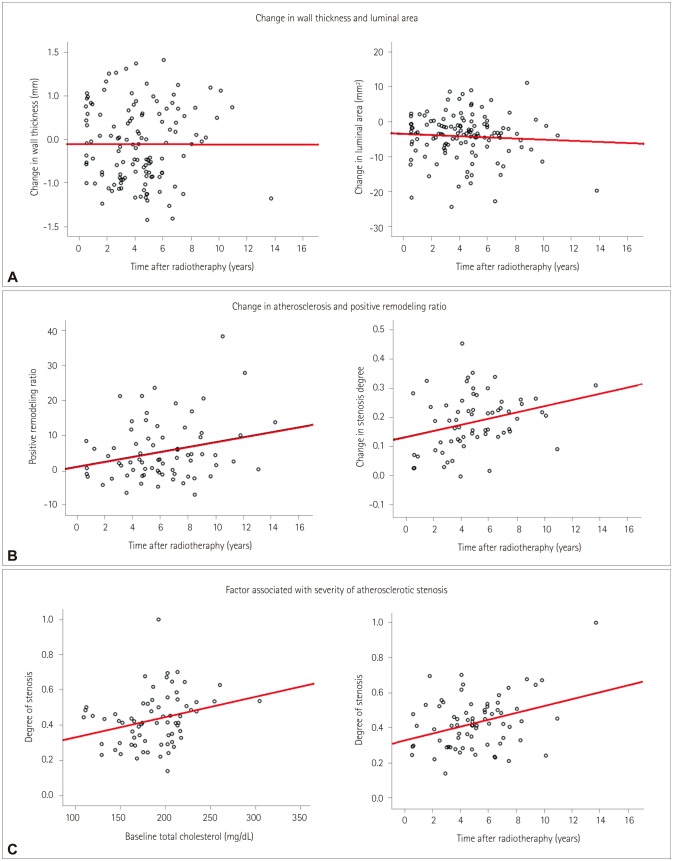
In total, 125 patients were enrolled. The wall thickness had increased and the lumen area had decreased several months after radiotherapy. These changes were not associated with vascular risk factors and were not progressive. Calcification and atherosclerosis were observed in 37 (29.6%) and 71 (56.8%) patients, respectively. Diabetes was associated with calcification (p=0.02). The prevalence of hyperlipidemia was higher in patients with atherosclerosis (28.2% vs. 11.1%, p=0.02) and for a longer period postradiation [62.7±32.1 vs. 40.0±24.2 months (mean±SD), p < 0.001]. Atherosclerosis occurred mostly in the middle portion of the CCA (n=31, 24.6%), followed by the proximal CCA at the intrathoracic level (n=26, 20.6%) and the distal CCA (n=6, 4.8%). Positive remodeling was also observed, but this was less common in patients with calcification (p=0.02).
CONCLUSIONS
Various types of postradiation changes occur in the CCA and can be easily observed in postradiation CT. The prevalence and burden of postradiation atherosclerosis increased in a close relationship with baseline cholesterol levels and the time after radiotherapy. Postradiation atherosclerosis was observed at unusual sites of the CCA.
MeSH Terms
Figure
Reference
-
1. Pfister DG, Laurie SA, Weinstein GS, Mendenhall WM, Adelstein DJ, et al. American Society of Clinical Oncology Clinical Practice Guideline for the use of larynx-preservation strategies in the treatment of laryngeal cancer. J Clin Oncol. 2006; 24:3693–3704. PMID: 16832122.
Article2. Forastiere AA, Goepfert H, Maor M, Pajak TF, Weber R, Morrison W, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003; 349:2091–2098. PMID: 14645636.
Article3. Carmody BJ, Arora S, Avena R, Curry KM, Simpkins J, Cosby K, et al. Accelerated carotid artery disease after high-dose head and neck radiotherapy: is there a role for routine carotid duplex surveillance? J Vasc Surg. 1999; 30:1045–1051. PMID: 10587388.
Article4. Kim K, Lee JH. Risk factors and biomarkers of ischemic stroke in cancer patients. J Stroke. 2014; 16:91–96. PMID: 24949315.
Article5. Plummer C, Henderson RD, O'Sullivan JD, Read SJ. Ischemic stroke and transient ischemic attack after head and neck radiotherapy: a review. Stroke. 2011; 42:2410–2418. PMID: 21817150.6. Weintraub NL, Jones WK, Manka D. Understanding radiation-induced vascular disease. J Am Coll Cardiol. 2010; 55:1237–1239. PMID: 20298931.
Article7. Dorresteijn LD, Kappelle AC, Scholz NM, Munneke M, Scholma JT, Balm AJ, et al. Increased carotid wall thickening after radiotherapy on the neck. Eur J Cancer. 2005; 41:1026–1230. PMID: 15862751.
Article8. Gujral DM, Shah BN, Chahal NS, Senior R, Harrington KJ, Nutting CM. Clinical features of radiation-induced carotid atherosclerosis. Clin Oncol (R Coll Radiol). 2014; 26:94–102. PMID: 24188597.
Article9. Amarenco P, Bogousslavsky J, Callahan A 3rd, Goldstein LB, Hennerici M, Rudolph AE, et al. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006; 355:549–559. PMID: 16899775.
Article10. Paraskevas KI, Hamilton G, Mikhailidis DP. Statins: an essential component in the management of carotid artery disease. J Vasc Surg. 2007; 46:373–386. PMID: 17664116.
Article11. Yamagami H, Sakaguchi M, Furukado S, Hoshi T, Abe Y, Hougaku H, et al. Statin therapy increases carotid plaque echogenicity in hypercholesterolemic patients. Ultrasound Med Biol. 2008; 34:1353–1359. PMID: 18378381.
Article12. Oliver TB, Lammie GA, Wright AR, Wardlaw J, Patel SG, Peek R, et al. Atherosclerotic plaque at the carotid bifurcation: CT angiographic appearance with histopathologic correlation. AJNR Am J Neuroradiol. 1999; 20:897–901. PMID: 10369363.13. Hardie AD, Kramer CM, Raghavan P, Baskurt E, Nandalur KR. The impact of expansive arterial remodeling on clinical presentation in carotid artery disease: a multidetector CT angiography study. AJNR Am J Neuroradiol. 2007; 28:1067–1070. PMID: 17569959.
Article14. Chang YJ, Chang TC, Lee TH, Ryu SJ. Predictors of carotid artery stenosis after radiotherapy for head and neck cancers. J Vasc Surg. 2009; 50:280–285. PMID: 19631860.
Article15. Li M, Wu SW, Xu WH. High-resolution MRI of radiation-induced intracranial vasculopathy. Neurology. 2015; 84:631. PMID: 25666631.
Article16. Virmani R, Farb A, Carter AJ, Jones RM. Pathology of radiation-induced coronary artery disease in human and pig. Cardiovasc Radiat Med. 1999; 1:98–101. PMID: 11272363.
Article17. Wu XH, Chen XY, Wang LJ, Wong KS. Intracranial artery calcification and its clinical significance. J Clin Neurol. 2016; 12:253–261. PMID: 27165425.
Article18. Demer LL, Tintut Y. Vascular calcification: pathobiology of a multifaceted disease. Circulation. 2008; 117:2938–2948. PMID: 18519861.19. Gujral DM, Chahal N, Senior R, Harrington KJ, Nutting CM. Radiation-induced carotid artery atherosclerosis. Radiother Oncol. 2014; 110:31–38. PMID: 24044796.
Article20. Higashikuni Y, Tanabe K, Yamamoto H, Aoki J, Nakazawa G, Onuma Y, et al. Relationship between coronary artery remodeling and plaque composition in culprit lesions: an intravascular ultrasound radiofrequency analysis. Circ J. 2007; 71:654–660. PMID: 17456987.21. Pereira EB, Gemignani T, Sposito AC, Matos-Souza JR, Nadruz W Jr. Low-density lipoprotein cholesterol and radiotherapy-induced carotid atherosclerosis in subjects with head and neck cancer. Radiat Oncol. 2014; 9:134. PMID: 24919963.
Article22. Wilbers J, Hoebers FJ, Boogerd W, van Werkhoven ED, Nowee ME, Hart G, et al. Prospective cohort study of carotid intima-media thickness after irradiation. J Stroke Cerebrovasc Dis. 2014; 23:2701–2707. PMID: 25304721.
Article23. Moon GJ, Kim SJ, Cho YH, Ryoo S, Bang OY. Antioxidant effects of statins in patients with atherosclerotic cerebrovascular disease. J Clin Neurol. 2014; 10:140–147. PMID: 24829600.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Association between Carotid Artery Intima-Media Thickness and Stroke Risk Factors in Ischemic Stroke
- Carotid ultrasound in patients with coronary artery disease
- Correlation between Intima-Media Thickness in Carotid Artery and the Extent of Coronary Atherosclerosis
- The Serum Lipid Level is Associated with Intimal Thickness of the Carotid Artery for Patients with Coronary Atherosclerosis
- Intima-Media Thickness of the Carotid Artery: non-invasive marker of atherosclerosis