Korean J Gastroenterol.
2017 Nov;70(5):239-246. 10.4166/kjg.2017.70.5.239.
Effect of Rifaximin on Hepatic Fibrosis in Bile Duct-ligated Rat Model
- Affiliations
-
- 1Division of Gastroenterology, Department of Internal Medicine, Gachon University Gil Medical Center, Incheon, Korea. kos@gilhospital.com
- 2Department of Surgery, Gachon University Gil Medical Center, Incheon, Korea.
- 3Division of Endocrinology, Department of Internal Medicine, Gachon University Gil Medical Center, Incheon, Korea.
- 4Division of Pulmonology, Department of Internal Medicine, Gachon University Gil Medical Center, Incheon, Korea.
- KMID: 2398870
- DOI: http://doi.org/10.4166/kjg.2017.70.5.239
Abstract
- BACKGROUND/AIMS
The translocation of bacteria and their lipopolysaccharides from the gut can promote fibrosis in cirrhotic patients. The aim of this study was to investigate the effects of rifaximin on hepatic fibrosis in a bile duct-ligated rat model.
METHODS
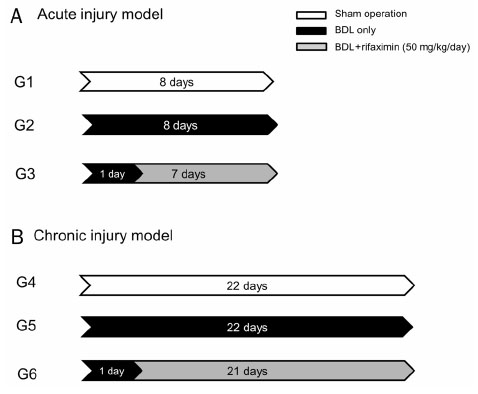
The bile duct ligation (BDL) was carried out for eight days (acute injury model: sham-operated rats [G1], BDL rats [G2], and BDL rats treated with rifaximin [G3]) or 22 days (chronic injury model: sham-operated rats [G4], BDL rats [G5], and BDL rats treated with rifaximin [G6]). Rifaximin (50 mg/kg/day) was administered daily via gavage after BDL. Liver function, serum tumor necrosis factor-alpha (TNF-α), and hepatic hydroxyproline levels were measured. Moreover, a histological analysis of fibrosis contents was performed using sirius red stain.
RESULTS
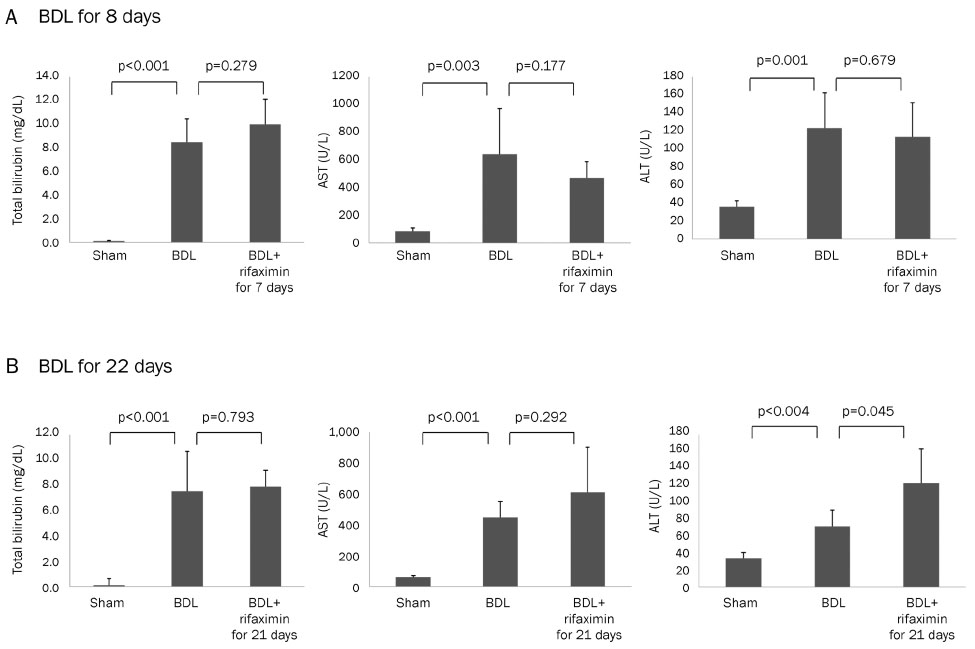
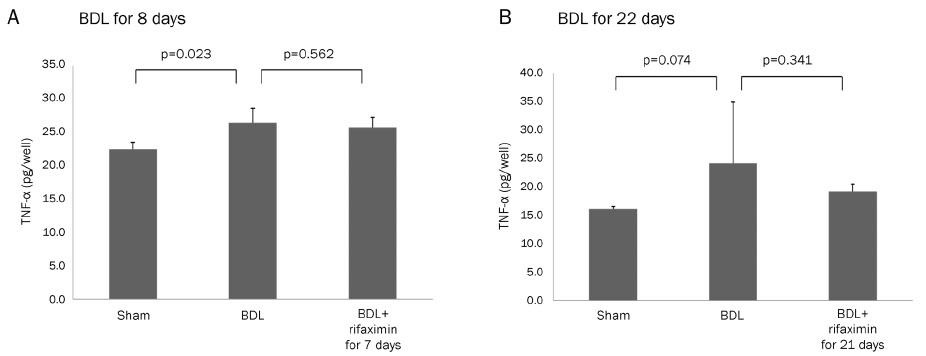
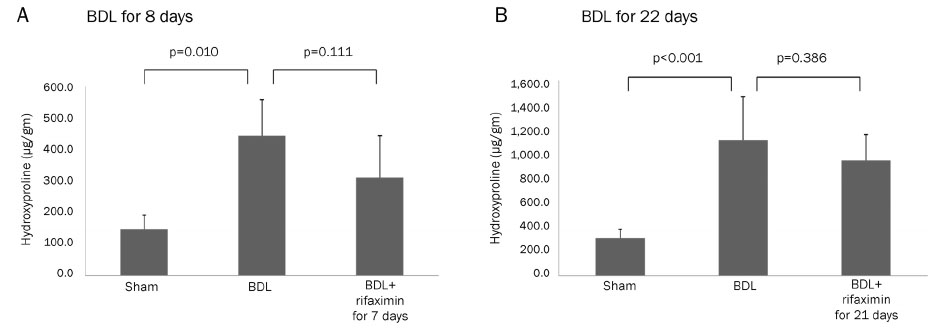
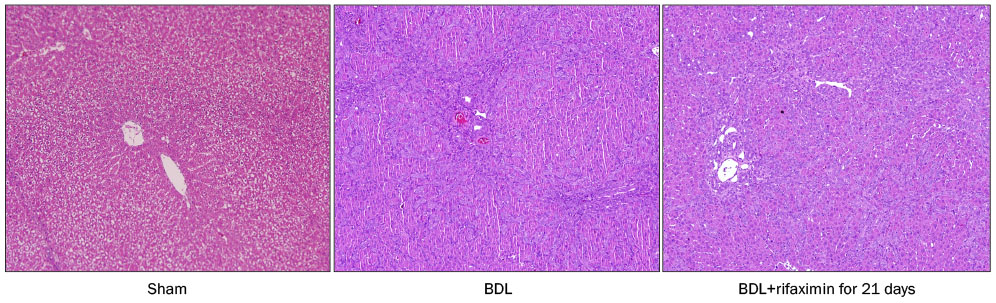
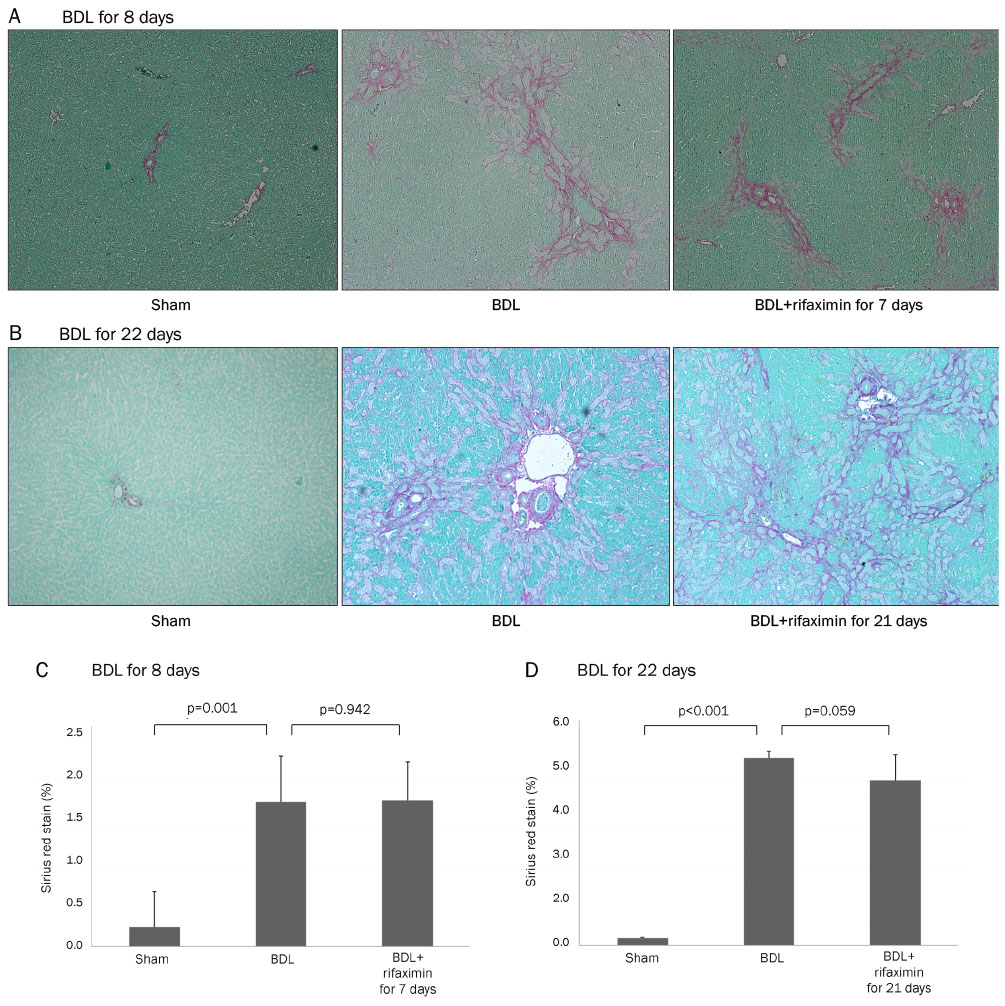
In the acute injury model, the liver function and TNF-α level were not improved after the rifaximin treatment. The hydroxyproline levels (µg/g liver tissue) in G1, G2, and G3 were 236.4±103.1, 444.8±114.4, and 312.5±131.6, respectively; and fibrosis contents (%) were 0.22±0.04, 1.64±0.53, and 1.66±0.44, respectively. The rifaximin treatment did not ameliorate acute BDL-induced fibrosis. In the chronic injury model, the hydroxyproline levels in G4, G5, and G6 were 311.5±72.9, 1,110.3±357.9, and 944.3±209.3, respectively; and fibrosis contents (%) were 0.19±0.03, 5.04±0.18, and 4.42±0.68, respectively (G5 vs. G6, p=0.059). The rifaximin treatment marginally ameliorated chronic BDL-induced fibrosis.
CONCLUSIONS
Rifaximin did not reduce inflammation and fibrosis in bile duct-ligated rat model.
Keyword
MeSH Terms
Figure
Reference
-
1. Benyon RC, Iredale JP. Is liver fibrosis reversible? Gut. 2000; 46:443–446.2. Alcolado R, Arthur MJ, Iredale JP. Pathogenesis of liver fibrosis. Clin Sci (Lond). 1997; 92:103–112.3. Schuppan D, Afdhal NH. Liver cirrhosis. Lancet. 2008; 371:838–851.4. Seki E, Brenner DA. Toll-like receptors and adaptor molecules in liver disease: update. Hepatology. 2008; 48:322–335.5. Crispe IN. The liver as a lymphoid organ. Annu Rev Immunol. 2009; 27:147–163.6. Pradere JP, Troeger JS, Dapito DH, Mencin AA, Schwabe RF. Toll-like receptor 4 and hepatic fibrogenesis. Semin Liver Dis. 2010; 30:232–244.7. Seki E, Schnabl B. Role of innate immunity and the microbiota in liver fibrosis: crosstalk between the liver and gut. J Physiol. 2012; 590:447–458.8. Kalambokis GN, Tsianos EV. Rifaximin reduces endotoxemia and improves liver function and disease severity in patients with decompensated cirrhosis. Hepatology. 2012; 55:655–656.9. Zhu Q, Zou L, Jagavelu K, et al. Intestinal decontamination inhibits TLR4 dependent fibronectin-mediated cross-talk between stellate cells and endothelial cells in liver fibrosis in mice. J Hepatol. 2012; 56:893–899.10. Yang L, Chan CC, Kwon OS, et al. Regulation of peroxisome proliferator-activated receptor-gamma in liver fibrosis. Am J Physiol-Gastrointest Liver Physiol. 2006; 291:G902–G911.11. Tarcin O, Basaranoglu M, Tahan V, et al. Time course of collagen peak in bile duct-ligated rats. BMC Gastroenterol. 2011; 11:45.12. Kwon OS, Choi SH, Kim JH. Inflammation and hepatic fibrosis, then hepatocellular carcinoma. Korean J Gastroenterol. 2015; 66:320–324.13. Paik YH, Schwabe RF, Bataller R, Russo MP, Jobin C, Brenner DA. Toll-like receptor 4 mediates inflammatory signaling by bacterial lipopolysaccharide in human hepatic stellate cells. Hepatology. 2003; 37:1043–1055.14. Guo J, Friedman SL. Toll-like receptor 4 signaling in liver injury and hepatic fibrogenesis. Fibrogenesis Tissue Repair. 2010; 3:21.15. Dawiskiba J, Kornafel P, Kwiatkowska D, Zimecki M. Alterations of tumor necrosis factor-alpha and interleukin 6 production and activity of the reticuloendothelial system in experimental obstructive jaundice in rats. HPB (Oxford). 2002; 4:11–19.16. Seki E, De Minicis S, Osterreicher CH, et al. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007; 13:1324–1332.17. Odena G, Miquel M, Serafín A, et al. Rifaximin, but not growth factor 1, reduces brain edema in cirrhotic rats. World J Gastroenterol. 2012; 18:2084–2091.18. Miglioli PA, Allerberger F, Calabrò GB, Gaion RM. Effects of daily oral administration of rifaximin and neomycin on faecal aerobic flora in rats. Pharmacol Res. 2001; 44:373–375.19. Vlachogiannakos J, Saveriadis AS, Viazis N, et al. Intestinal decontamination improves liver haemodynamics in patients with alcohol-related decompensated cirrhosis. Aliment Pharmacol Ther. 2009; 29:992–999.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunohistochemical Expression of Bcl-2 and Bax after Bile Duct Ligation in the Rat
- Ultrastructural and Immunohistochemical Study of Hepatic Fibrosis after the Ligation of the Common Bile Duct in Rats
- Effect of Hydrocortisone on Cytochrome P-450 Apoproteins in Bile Duct Ligated and Adrenalectomized Rats
- Effects of Exogenous Cholic Acid on Total Hepatic Cytochrome P-450 and b5 in the Bile Duct-ligated Rats
- Temporal Morphologic Changes in the Mouse Liver after Common Bile Duct Ligation