J Korean Assoc Pediatr Surg.
2017 Dec;23(2):42-47. 10.13029/jkaps.2017.23.2.42.
Thyroid Cancer in Pediatric Age: A Single Institution Experience
- Affiliations
-
- 1Department of Pediatric Surgery, Seoul National University Children's Hospital, Seoul, Korea.
- 2Department of Pediatric Surgery, Seoul National University College of Medicine, Seoul, Korea. spkhy02@snu.ac.kr
- 3Department of Surgery, Chung-Ang University College of Medicine, Seoul, Korea.
- KMID: 2398575
- DOI: http://doi.org/10.13029/jkaps.2017.23.2.42
Abstract
- PURPOSE
Thyroid cancer is a rare disease in pediatric population, but its incidence rate is increasing. The aim of this report is to present a single institution experience of pediatric thyroid cancer and to identify clinical features, predisposing factors, and postoperative course of pediatric thyroid cancer.
METHODS
We retrospectively reviewed 35 pediatric patients who underwent operation due to thyroid cancer at Seoul National University Children's Hospital between May 1997 and January 2017. The median follow-up period was 70 months (range, 5-238 months).
RESULTS
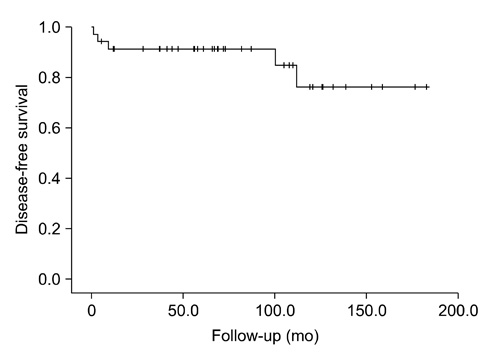
The mean age at operation was 12.0±5.91 years and 27 patients were female. The underlying conditions in patients included history of chemoradiotherapy for previous other malignancies (n=4), hypothyroidism (n=3), history of chemotherapy (n=2), family history of thyroid cancer (n=1) and history of radiation therapy (n=1). The initial symptoms were palpable neck mass (n=21) and incidental findings (n=11). Total thyroidectomy (n=30) or unilateral lobectomy (n=5) were performed. There were 15 postoperative complications including transient hypocalcemia in 14 patients and Horner's syndrome in 1 patient. The most common pathologic cell type was papillary thyroid cancer (n=29). Extrathyroid extension and lymph node invasion were found in 25 patients and 27 patients, respectively. Thirteen patients showed multifocality. During follow-up period, 5 patients underwent additional operation because of tumor recurrence in lymph nodes. Lung metastasis was detected in 3 patients at the time of diagnosis and in 3 patients during follow-up period. The mortality rate was zero and mean disease-free survival was 83.7±47.9 months.
CONCLUSION
Pediatric thyroid cancer has lower mortality rate and recurrence rate as seen in this study despite the advanced stage at diagnosis. A thorough follow-up of patients with an underlying condition such as history of chemoradiotherapy and understanding new pediatric guideline can be helpful to maximize patients' survival and prognosis.
Keyword
MeSH Terms
-
Causality
Chemoradiotherapy
Diagnosis
Disease-Free Survival
Drug Therapy
Female
Follow-Up Studies
Horner Syndrome
Humans
Hypocalcemia
Hypothyroidism
Incidence
Incidental Findings
Lung
Lymph Nodes
Mortality
Neck
Neoplasm Metastasis
Pediatrics
Postoperative Complications
Prognosis
Rare Diseases
Recurrence
Retrospective Studies
Seoul
Thyroid Gland*
Thyroid Neoplasms*
Thyroidectomy
Figure
Reference
-
1. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014; 64:9–29.2. Siegel DA, King J, Tai E, Buchanan N, Ajani UA, Li J. Cancer incidence rates and trends among children and adolescents in the United States, 2001-2009. Pediatrics. 2014; 134:e945–e955.3. Steliarova-Foucher E, Stiller CA, Pukkala E, Lacour B, Plesko I, Parkin DM. Thyroid cancer incidence and survival among European children and adolescents (1978-1997): report from the automated childhood cancer information system project. Eur J Cancer. 2006; 42:2150–2169.4. Cho YY, Jang HW, Joung JY, Park SM, Jeong DJ, Kim SW, et al. Trends in thyroid cancer incidence in Korean children (1999-2012) based on palpation and nonpalpation detection methods. Eur Thyroid J. 2015; 4:252–259.5. Oh CM, Won YJ, Jung KW, Kong HJ, Cho H, Lee JK, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2013. Cancer Res Treat. 2016; 48:436–450.6. McNally RJ, Blakey K, James PW, Gomez Pozo B, Basta NO, Hale J. Increasing incidence of thyroid cancer in Great Britain, 1976-2005: age-period-cohort analysis. Eur J Epidemiol. 2012; 27:615–622.7. Moon EK, Park HJ, Oh CM, Jung KW, Shin HY, Park BK, et al. Cancer incidence and survival among adolescents and young adults in Korea. PLoS One. 2014; 9:e96088.8. Czene K, Lichtenstein P, Hemminki K. Environmental and heritable causes of cancer among 9.6 million individuals in the Swedish family-cancer database. Int J Cancer. 2002; 99:260–266.9. Park YJ, Ahn HY, Choi HS, Kim KW, Park DJ, Cho BY. The long-term outcomes of the second generation of familial nonmedullary thyroid carcinoma are more aggressive than sporadic cases. Thyroid. 2012; 22:356–362.10. Williams ED, Doniach I, Bjarnason O, Michie W. Thyroid cancer in an iodide rich area: a histopathological study. Cancer. 1977; 39:215–222.11. Romei C, Fugazzola L, Puxeddu E, Frasca F, Viola D, Muzza M, et al. Modifications in the papillary thyroid cancer gene profile over the last 15 years. J Clin Endocrinol Metab. 2012; 97:E1758–E1765.12. Kazakov VS, Demidchik EP, Astakhova LN. Thyroid cancer after Chernobyl. Nature. 1992; 359:21.13. Furukawa K, Preston D, Funamoto S, Yonehara S, Ito M, Tokuoka S, et al. Long-term trend of thyroid cancer risk among Japanese atomic-bomb survivors: 60 years after exposure. Int J Cancer. 2013; 132:1222–1226.14. White MG, Cipriani NA, Abdulrasool L, Kaplan S, Aschebrook-Kilfoy B, Angelos P, et al. Radiation-induced differentiated thyroid cancer is associated with improved overall survival but not thyroid cancer-specific mortality or disease-free survival. Thyroid. 2016; 26:1053–1060.15. Sinnott B, Ron E, Schneider AB. Exposing the thyroid to radiation: a review of its current extent, risks, and implications. Endocr Rev. 2010; 31:756–773.16. Mazonakis M, Tzedakis A, Damilakis J, Gourtsoyiannis N. Thyroid dose from common head and neck CT examinations in children: is there an excess risk for thyroid cancer induction? Eur Radiol. 2007; 17:1352–1357.17. Francis GL, Waguespack SG, Bauer AJ, Angelos P, Benvenga S, Cerutti JM, et al. Management guidelines for children with thyroid nodules and differentiated thyroid cancer. Thyroid. 2015; 25:716–759.18. Welch Dinauer CA, Tuttle RM, Robie DK, McClellan DR, Svec RL, Adair C, et al. Clinical features associated with metastasis and recurrence of differentiated thyroid cancer in children, adolescents and young adults. Clin Endocrinol (Oxf). 1998; 49:619–628.19. Gupta A, Ly S, Castroneves LA, Frates MC, Benson CB, Feldman HA, et al. A standardized assessment of thyroid nodules in children confirms higher cancer prevalence than in adults. J Clin Endocrinol Metab. 2013; 98:3238–3245.20. Chow SM, Law SC, Mendenhall WM, Au SK, Yau S, Mang O, et al. Differentiated thyroid carcinoma in childhood and adolescence-clinical course and role of radioiodine. Pediatr Blood Cancer. 2004; 42:176–183.21. Harness JK, Thompson NW, McLeod MK, Pasieka JL, Fukuuchi A. Differentiated thyroid carcinoma in children and adolescents. World J Surg. 1992; 16:547–553. discussion 553-4.22. LaFranchi SH. Inaugural management guidelines for children with thyroid nodules and differentiated thyroid cancer: children are not small adults. Thyroid. 2015; 25:713–715.23. Handkiewicz-Junak D, Wloch J, Roskosz J, Krajewska J, Kropinska A, Pomorski L, et al. Total thyroidectomy and adjuvant radioiodine treatment independently decrease locoregional recurrence risk in childhood and adolescent differentiated thyroid cancer. J Nucl Med. 2007; 48:879–888.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Characteristics of Pediatric & Adolescent Thyroid Cancer: A Single Institution Experience of 20 Years
- Clinical experience with thyroid cancer in the pediatric age group
- Papillary Cancer of the Thyroid Gland in Childhood and Adolescence: A Review of 3 Patients
- Diagnosis and Treatment of Thyroid Nodules in Pediatric Age
- A 20 years, experience with well differentiated thyroid carcinoma in children & teenagers