J Vet Sci.
2017 Dec;18(4):487-497. 10.4142/jvs.2017.18.4.487.
KCHO-1, a novel herbal anti-inflammatory compound, attenuates oxidative stress in an animal model of amyotrophic lateral sclerosis
- Affiliations
-
- 1Adult Stem Cell Research Center, College of Veterinary Medicine, Seoul National University, Seoul 08826, Korea. kangpub@snu.ac.kr
- 2Research Institute for Veterinary Science, College of Veterinary Medicine, Seoul National University, Seoul 08826, Korea.
- 3Center of Integrative Medicine, Department of Internal Medicine, Wonkwang University Gwangju Hospital, Wonkwang University Gwangju Medical Center, Gwangju 61729, Korea.
- 4Department of Internal Medicine, School of Oriental Medicine, Wonkwang University, Iksan 54538, Korea.
- 5Department of Neurology, Inam Neuroscience Research Center, Wonkwang University Sanbon Hospital, Gunpo 15865, Korea.
- 6ALS/MND Center of Wonkwang University Korean Medical Hospital, Wonkwang University Gwangju Medical Center, Gwangju 61729, Korea. kangpub@snu.ac.kr
- KMID: 2398508
- DOI: http://doi.org/10.4142/jvs.2017.18.4.487
Abstract
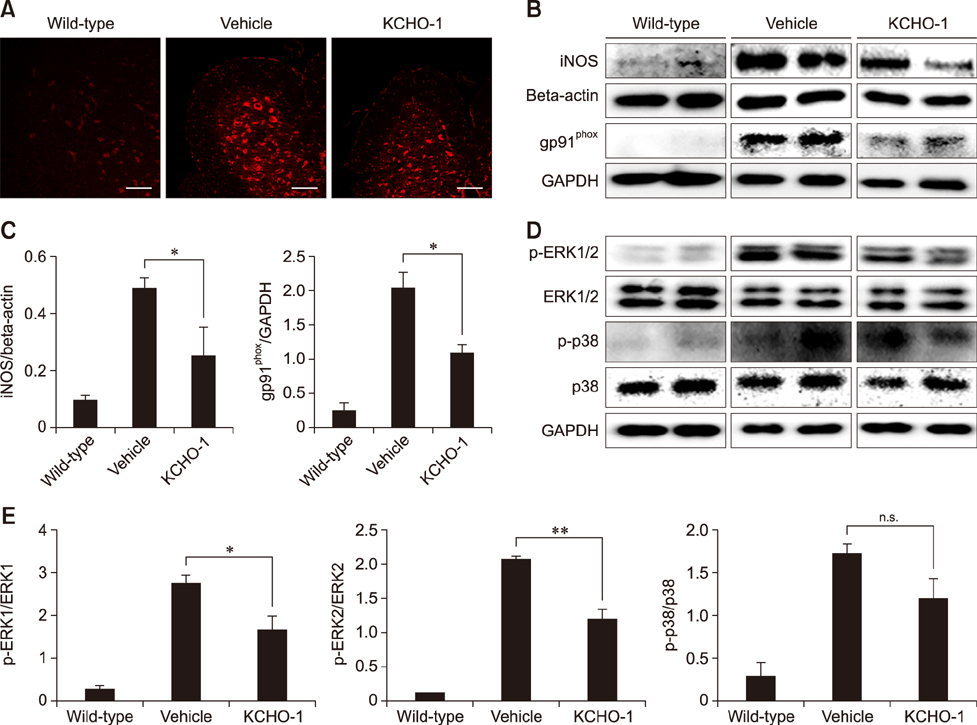
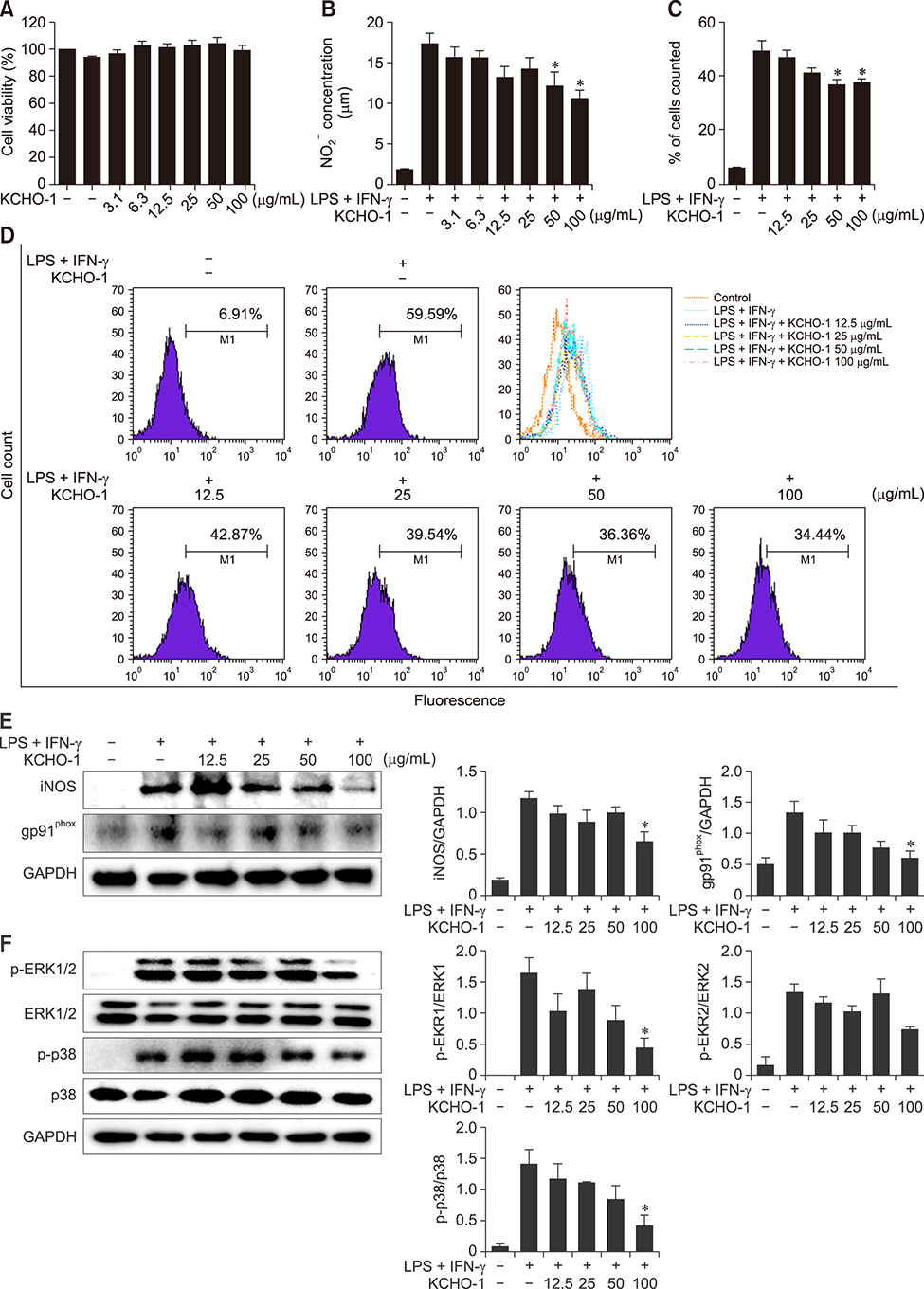
- Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease characterized by selective death of motor neurons in the central nervous system. The main cause of the disease remains elusive, but several mutations have been associated with the disease process. In particular, mutant superoxide dismutase 1 (SOD1) protein causes oxidative stress by activating glia cells and contributes to motor neuron degeneration. KCHO-1, a novel herbal combination compound, contains 30% ethanol and the extracts of nine herbs that have been commonly used in traditional medicine to prevent fatigue or inflammation. In this study, we investigated whether KCHO-1 administration could reduce oxidative stress in an ALS model. KCHO-1 administered to ALS model mice improved motor function and delayed disease onset. Furthermore, KCHO-1 administration reduced oxidative stress through gp91(phox) and the MAPK pathway in both classically activated microglia and the spinal cord of hSOD1(G93A) transgenic mice. The results suggest that KCHO-1 can function as an effective therapeutic agent for ALS by reducing oxidative stress.
Keyword
MeSH Terms
Figure
Reference
-
1. Apolloni S, Parisi C, Pesaresi MG, Rossi S, Carrì MT, Cozzolino M, Volonté C, D'Ambrosi N. The NADPH oxidase pathway is dysregulated by the P2X7 receptor in the SOD1-G93A microglia model of amyotrophic lateral sclerosis. J Immunol. 2013; 190:5187–5195.
Article2. Barber SC, Shaw PJ. Oxidative stress in ALS: key role in motor neuron injury and therapeutic target. Free Radic Biol Med. 2010; 48:629–641.
Article3. Ben Haim L, Carrillo-de Sauvage MA, Ceyzériat K, Escartin C. Elusive roles for reactive astrocytes in neurodegenerative diseases. Front Cell Neurosci. 2015; 9:278.
Article4. Boillée S, Cleveland DW. Revisiting oxidative damage in ALS: microglia, Nox, and mutant SOD1. J Clin Invest. 2008; 118:474–478.
Article5. Bordt EA, Polster BM. NADPH oxidase- and mitochondria-derived reactive oxygen species in proinflammatory microglial activation: a bipartisan affair? Free Radic Biol Med. 2014; 76:34–46.
Article6. Bozzo F, Mirra A, Carrì MT. Oxidative stress and mitochondrial damage in the pathogenesis of ALS: new perspectives. Neurosci Lett. 2017; 636:3–8.
Article7. Brown GC, Neher JJ. Inflammatory neurodegeneration and mechanisms of microglial killing of neurons. Mol Neurobiol. 2010; 41:242–247.
Article8. Bucchia M, Ramirez A, Parente V, Simone C, Nizzardo M, Magri F, Dametti S, Corti S. Therapeutic development in amyotrophic lateral sclerosis. Clin Ther. 2015; 37:668–680.
Article9. Cardiff RD, Miller CH, Munn RJ. Manual hematoxylin and eosin staining of mouse tissue sections. Cold Spring Harb Protoc. 2014; 2014:655–658.
Article10. Du H, Wu J, Li H, Zhong PX, Xu YJ, Li CH, Ji KX, Wang LS. Polyphenols and triterpenes from Chaenomeles fruits: chemical analysis and antioxidant activities assessment. Food Chem. 2013; 141:4260–4268.
Article11. Hong JA, Chung SH, Lee JS, Kim SS, Shin HD, Kim H, Jang MH, Lee TH, Lim BV, Kim YP, Kim CJ. Effects of Paeonia radix on 5-hydroxytryptamine synthesis and tryptophan hydroxylase expression in the dorsal raphe of exercised rats. Biol Pharm Bull. 2003; 26:166–169.
Article12. Hong MH, Kim JH, Bae H, Lee NY, Shin YC, Kim SH, Ko SG. Atractylodes japonica Koidzumi inhibits the production of proinflammatory cytokines through inhibition of the NF-κB/IκB signal pathway in HMC-1 human mast cells. Arch Pharm Res. 2010; 33:843–851.
Article13. Hooten KG, Beers DR, Zhao W, Appel SH. Protective and toxic neuroinflammation in amyotrophic lateral sclerosis. Neurotherapeutics. 2015; 12:364–375.
Article14. Jeong H, Lee J, Cha E, Park M, Son I, Song B, Kim S. A study on the oral toxicity of mecasin in rats. J Pharmacopuncture. 2014; 17:61–65.
Article15. Jiang WY, Jeon BH, Kim YC, Lee SH, Sohn DH, Seo GS. PF2401-SF, standardized fraction of Salvia miltiorrhiza shows anti-inflammatory activity in macrophages and acute arthritis in vivo. Int Immunopharmacol. 2013; 16:160–164.
Article16. Kim BW, Koppula S, Kim JW, Lim HW, Hwang JW, Kim IS, Park PJ, Choi DK. Modulation of LPS-stimulated neuroinflammation in BV-2 microglia by Gastrodia elata: 4-hydroxybenzyl alcohol is the bioactive candidate. J Ethnopharmacol. 2012; 139:549–557.
Article17. Kim EK, Choi EJ. Pathological roles of MAPK signaling pathways in human diseases. Biochim Biophys Acta. 2010; 1802:396–405.
Article18. Kim KS, Lee DS, Bae GS, Park SJ, Kang DG, Lee HS, Oh H, Kim YC. The inhibition of JNK MAPK and NF-kappaB signaling by tenuifoliside A isolated from Polygala tenuifolia in lipopolysaccharide-induced macrophages is associated with its anti-inflammatory effect. Eur J Pharmacol. 2013; 721:267–276.
Article19. Kim Y, You Y, Yoon HG, Lee YH, Kim K, Lee J, Kim MS, Kim JC, Jun W. Hepatoprotective effects of fermented Curcuma longa L. on carbon tetrachloride-induced oxidative stress in rats. Food Chem. 2014; 151:148–153.
Article20. Kobayashi K, Imagama S, Ohgomori T, Hirano K, Uchimura K, Sakamoto K, Hirakawa A, Takeuchi H, Suzumura A, Ishiguro N, Kadomatsu K. Minocycline selectively inhibits M1 polarization of microglia. Cell Death Dis. 2013; 4:e525.
Article21. Kwiatkowski TJ Jr, Bosco DA, LeClerc AL, Tamrazian E, Vanderburg CR, Russ C, Davis A, Gilchrist J, Kasarskis EJ, Munsat T, Valdmanis P, Rouleau GA, Hosler BA, Cortelli P, de Jong PJ, Yoshinaga Y, Haines JL, Pericak-Vance MA, Yan J, Ticozzi N, Siddique T, McKenna-Yasek D, Sapp PC, Horvitz HR, Landers JE, Brown RH Jr. Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science. 2009; 323:1205–1208.
Article22. Lee DS, Ko W, Song BK, Son I, Kim DW, Kang DG, Lee HS, Oh H, Jang JH, Kim YC, Kim S. The herbal extract KCHO-1 exerts a neuroprotective effect by ameliorating oxidative stress via heme oxygenase-1 upregulation. Mol Med Rep. 2016; 13:4911–4919.
Article23. Lee DS, Ko W, Yoon CS, Kim DC, Yun J, Lee JK, Jun KY, Son I, Kim DW, Song BK, Choi S, Jang JH, Oh H, Kim S, Kim YC. KCHO-1, a novel antineuroinflammatory agent, inhibits lipopolysaccharide-induced neuroinflammatory responses through Nrf2-mediated heme oxygenase-1 expression in mouse BV2 microglia cells. Evid Based Complement Alternat Med. 2014; 2014:357154.
Article24. Liao B, Zhao W, Beers DR, Henkel JS, Appel SH. Transformation from a neuroprotective to a neurotoxic microglial phenotype in a mouse model of ALS. Exp Neurol. 2012; 237:147–152.
Article25. Manavalan A, Ramachandran U, Sundaramurthi H, Mishra M, Sze SK, Hu JM, Feng ZW, Heese K. Gastrodia elata Blume (tianma) mobilizes neuro-protective capacities. Int J Biochem Mol Biol. 2012; 3:219–241.26. Marden JJ, Harraz MM, Williams AJ, Nelson K, Luo M, Paulson H, Engelhardt JF. Redox modifier genes in amyotrophic lateral sclerosis in mice. J Clin Invest. 2007; 117:2913–2919.
Article27. Mungrue IN, Husain M, Stewart DJ. The role of NOS in heart failure: lessons from murine genetic models. Heart Fail Rev. 2002; 7:407–422.
Article28. Murdock BJ, Bender DE, Segal BM, Feldman EL. The dual roles of immunity in ALS: injury overrides protection. Neurobiol Dis. 2015; 77:1–12.
Article29. Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, McCluskey LF, Miller BL, Masliah E, Mackenzie IR, Feldman H, Feiden W, Kretzschmar HA, Trojanowski JQ, Lee VMY. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006; 314:130–133.
Article30. Palomo GM, Manfredi G. Exploring new pathways of neurodegeneration in ALS: the role of mitochondria quality control. Brain Res. 2015; 1607:36–46.
Article31. Pasinelli P, Brown RH. Molecular biology of amyotrophic lateral sclerosis: insights from genetics. Nat Rev Neurosci. 2006; 7:710–723.
Article32. Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, Donaldson D, Goto J, O'Regan JP, Deng HX, Rahmani Z, Krizus A, McKennna-Yasek D, Cayabyab A, Gaston SM, Berger R, Tanzi RE, Halperin JJ, Herzfeldt B, Van den Bergh R, Hung WY, Bird T, Deng G, Mulder DW, Smyth C, Laing NG, Soriano E, Pericak-Vance MA, Haines J, Rouleau GA, Gusella JS, Horvitz HR, Brown RH Jr. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993; 362:59–62.
Article33. Sancheti S, Sancheti S, Seo SY. Antidiabetic and antiacetylcholinesterase effects of ethyl acetate fraction of Chaenomeles sinensis (Thouin) Koehne fruits in streptozotocin-induced diabetic rats. Exp Toxicol Pathol. 2013; 65:55–60.
Article34. Takeuchi A, Isobe KI, Miyaishi O, Sawada M, Fan ZH, Nakashima I, Kiuchi K. Microglial NO induces delayed neuronal death following acute injury in the striatum. Eur J Neurosci. 1998; 10:1613–1620.
Article35. Villinski JR, Bergeron C, Cannistra JC, Gloer JB, Coleman CM, Ferreira D, Azelmat J, Grenier D, Gafner S. Pyranoisoflavans from Glycyrrhiza uralensis with antibacterial activity against Streptococcus mutans and Porphyromonas gingivalis. J Nat Prod. 2014; 77:521–526.
Article36. Wu DC, Ré DB, Nagai M, Ischiropoulos H, Przedborski S. The inflammatory NADPH oxidase enzyme modulates motor neuron degeneration in amyotrophic lateral sclerosis mice. Proc Natl Acad Sci U S A. 2006; 103:12132–12137.
Article37. Xu H, Arita H, Hayashida M, Zhang L, Sekiyama H, Hanaoka K. Pain-relieving effects of processed Aconiti tuber in CCI-neuropathic rats. J Ethnopharmacol. 2006; 103:392–397.
Article38. Yang EJ, Jiang JH, Lee SM, Yang SC, Hwang HS, Lee MS, Choi SM. Bee venom attenuates neuroinflammatory events and extends survival in amyotrophic lateral sclerosis models. J Neuroinflammation. 2010; 7:69.
Article39. Zhang F, Jiang L. Neuroinflammation in Alzheimer's disease. Neuropsychiatr Dis Treat. 2015; 11:243–256.40. Zhao W, Beers DR, Appel SH. Immune-mediated mechanisms in the pathoprogression of amyotrophic lateral sclerosis. J Neuroimmune Pharmacol. 2013; 8:888–899.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Amyotrophic Lateral Sclerosis Associated With CADASIL
- Molecular factors related to skeletal muscle atrophy in a mouse model of amyotrophic lateral sclerosis
- Syndrome of Progressive Bulbar Palsy in Amyotrophic Lateral Sclerosis: A Case Report
- Apraxia of Eyelid Closure and Motor Impersistence of Eyelid in a Patient with Amyotrophic Lateral Sclerosis
- Diagnosis and Therapeutic Strategies of Amyotrophic Lateral Sclerosis