J Vet Sci.
2017 Dec;18(4):457-464. 10.4142/jvs.2017.18.4.457.
Pathogenesis of coxsackievirus B2 in mice: characterization of clinical isolates of the coxsackievirus B2 from patients with myocarditis and aseptic meningitis in Korea
- Affiliations
-
- 1Laboratory Animal Medicine, College of Veterinary Medicine, Seoul National University, Seoul 08826, Korea. pjhak@snu.ac.kr
- 2Vaccines Division, National Institute of Food & Drug Safety Evaluation, Ministry of Food and Drug Safety, Cheongju 28159, Korea.
- 3Division of Vaccine Research, Center for Infectious Disease, National Institute of Health, Korea Centers for Disease Control and Prevention, Cheongju 28159, Korea.
- KMID: 2398504
- DOI: http://doi.org/10.4142/jvs.2017.18.4.457
Abstract
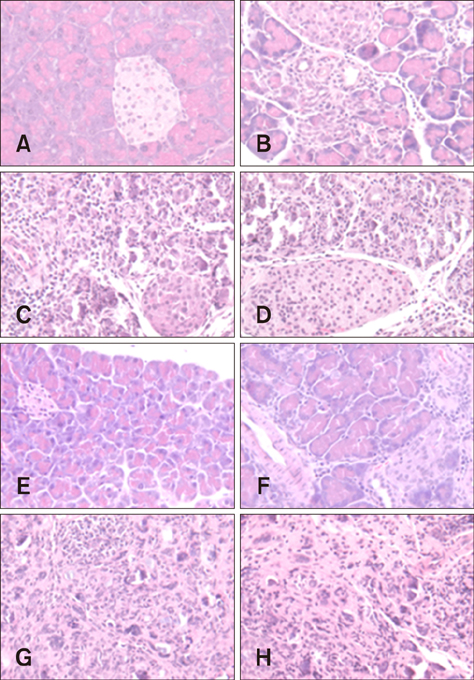
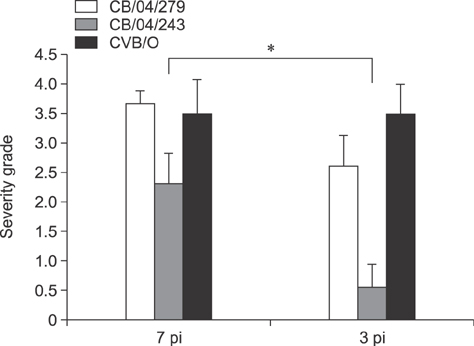
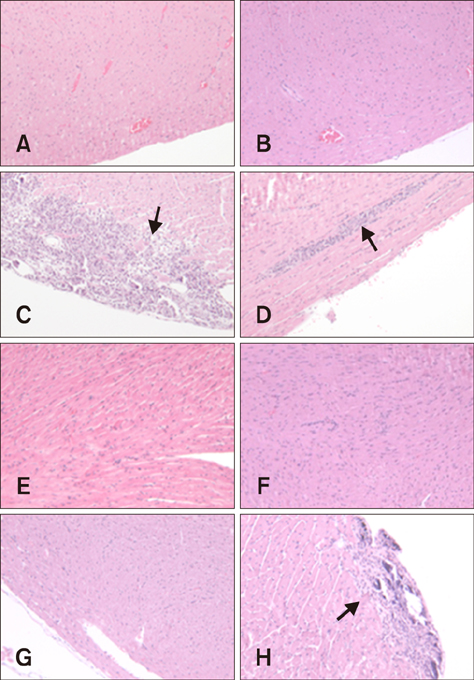
- Group B coxsackieviruses (CVBs) are a group of common human pathogens producing various clinical symptoms. Although the virology of CVB is well known, there is limited information on viral pathogenesis and the relationship between clinical symptoms and viral phenotype, particularly for CVB type 2 (CVB2). In 2004 in Korea, two CVB2 strains were isolated: CB2/04/279 from stool of an acute myocarditis patient with heart failure and CB2/04/243 from an aseptic meningitis patient. In this study, a high degree of homology was observed between the CB2/04/279 and CB2/04/243 full genome sequences. The two Korean CVB2 isolates had 93.1% homology compared to 82.1%-82.5% nucleotide sequence identity with the cardiovirulence-associated reference CVB strain Ohio-1 (CVB/O). CVB2-induced pathogenesis was analyzed, focusing on virus-induced pathology of various tissues in 4-week-old BALB/c inbred male mice. Myocarditis developed and extensive pancreatic inflammation was observed in all mice infected with CB2/04/279 or CVB/O, but not in animals infected with CB2/04/243. This is the first report of the full-genomic sequence and pathogenesis of the CVB2 strain isolated from an acute myocarditis patient in Korea.
Keyword
MeSH Terms
Figure
Reference
-
1. Ahn J, Chio J, Joo CH, Seo I, Kim D, Yoon SY, Kim YK, Lee H. Susceptibility of mouse primary cortical neuronal cells to coxsackievirus B. J Gen Virol. 2004; 85:1555–1564.
Article2. Baboonian C, Treasure T. Meta-analysis of the association of enteroviruses with human heart disease. Heart. 1997; 78:539–543.
Article3. Badorff C, Knowlton KU. Dystrophin disruption in enterovirus-induced myocarditis and dilated cardiomyopathy: from bench to bedside. Med Microbiol Immunol. 2004; 193:121–126.
Article4. Bowles NE, Richardson PJ, Olsen EGJ, Archard LC. Detection of coxsackie-B-virus-specific RNA sequences in myocardial biopsies samples from patients with myocarditis and dilated cardiomyopathy. Lancet. 1986; 1:1120–1123.5. Chiou CC, Liu WT, Chen SJ, Soong WJ, Wu KG, Tang RB, Hwang B. Coxsackievirus B1 infection in infants less than 2 months of age. Am J Perinatol. 1998; 15:155–159.
Article6. Dalldorf G, Melnick JL, Curnen EC. The coxsackie virus group. In : Rivers TM, Horsfall FL, editors. Viral and Rickettsial Infections of Man. 3rd ed. Philadelphia: Lippinocott;1959. p. 519–546.7. Dunn JJ, Bradrick SS, Chapman NM, Tracy S, Romero JR. The stem loop II within the 5′ nontranslated region of clinical coxsackievirus B3 genomes determines cardiovirulence phenotype in a murine model. J Infect Dis. 2003; 187:1552–1561.
Article8. Dunn JJ, Chapman NM, Tracy S, Romero JR. Genomic determinants of cardiovirulence in coxsackievirus B3 clinical isolates: localization to the 5′ nontranslated region. J Virol. 2000; 74:4787–4794.
Article9. Griffin DE, Hardwick JM. Perspective: virus infections and the death of neurons. Trends Microbiol. 1999; 7:155–160.
Article10. Jenista JA, Powell KR, Menegus MA. Epidemiology of neonatal enterovirus infection. J Pediatr. 1984; 104:685–690.
Article11. Johnson RT. Meningitis, encephalitis, and poliomyelitis. Viral Infections of the Nervous System. Philadelphia: Lippincott-Raven;1998. p. 87–132.12. Kim KS, Hufnagel G, Chapman NM, Tracy S. The group B coxsackieviruses and myocarditis. Rev Med Virol. 2001; 11:355–368.
Article13. Kim KS, Tracy S, Tapprich W, Bailey J, Lee CK, Kim K, Barry WH, Chapman NM. 5′-Terminal deletions occur in coxsackievirus B3 during replication in murine hearts and cardiac myocyte cultures and correlate with encapsidation of negative-strand viral RNA. J Virol. 2005; 79:7024–7041.
Article14. Liapounova NA, Mouquet F, Ennezat PV. Acute myocardial infarction spurred by myopericarditis in a young female patient: coxsackie B2 to blame. Acta Cardiol. 2011; 66:79–81.
Article15. Melnick JL. Enteroviruses: polioviruses, coxsackieviruses, echoviruses, and newer enteroviruses. In : Fields BN, Knipe DM, Howley PM, editors. Fields Virology. 3th ed. Philadelphia: Lippincott-Raven;1996. p. 655–712.16. Melnick JL, Ledinko N, Kaplan AS, Kraft LM. Ohio strains of virus pathogenic for infant mice (coxsackie group), simultaneous occurrence with poliomyelitis virus in patients with “summer grippe”. J Exp Med. 1950; 91:185–195.
Article17. Muckelbauer JK, Kremer M, Minor I, Diana G, Dutko FJ, Groarke J, Pevear DC, Rossmann MG. The structure of coxsackievirus B3 at 3.5 Å resolution. Structure. 1995; 3:653–667.
Article18. Minor PD, Ferguson M, Evans DMA, Almond JW, Icenogle JP. Antigenic structure of polioviruses of serotypes 1, 2 and 3. J Gen Virol. 1986; 67:1283–1291.
Article19. Polacek C, Lindberg AM. Genetic characterization of the coxsackievirus B2 3′ untranslated region. J Gen Virol. 2001; 82:1339–1348.
Article20. Polacek C, Lundgren A, Andersson A, Lindberg AM. Genomic and phylogenetic characterization of coxsackievirus B2 prototype strain Ohio-1. Virus Res. 1999; 59:229–238.
Article21. Rueckert RR. Picornaviridae: the viruses and their replication. In : Fields BN, Knipe DM, Howley PM, editors. Fields Virology. 3th ed. Philadelphia: Lippincott-Raven;1996. p. 609–654.22. Sawyer MH. Enterovirus infections: diagnosis and treatment. Pediatr Infect Dis J. 1999; 18:1033–1040.
Article23. Selinka HC, Wolde A, Sauter M, Kandolf R, Klingel K. Virus-receptor interactions of coxsackie B viruses and their putative influence on cardiotropism. Med Microbiol Immunol. 2004; 193:127–131.
Article24. Sherry B, Mosser AG, Colonno RJ, Rueckert RR. Use of monoclonal antibodies to identify four neutralizing immunogens on a commom cold picornavirus, human rhinovirus 14. J Virol. 1986; 57:246–257.
Article25. Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994; 22:4673–4680.
Article26. Towner JS, Ho TV, Semler BL. Determinants of membrane association for poliovirus protein 3AB. J Biol Chem. 1996; 271:26810–26818.
Article27. Woodruff JF. Viral myocarditis. A review. Am J Pathol. 1980; 101:425–484.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Isolation of Causative Viruses from Patients with Aseptic Meningitis in Gwangju Area
- Coxsackievirus A16 Isolated from Patients with Hand-Foot-Mouth Disease in Cheiu Province in the Spring of 1998
- Aseptic meningitis in 1991: isolation of causative agent
- Serotyping and Phylogenetic analysis of Enteroviruses Isolated from Patients with Aspetic Meningitis
- Characterization of Enteroviruses Isolated from Patients with Aseptic Meningitis