J Breast Cancer.
2017 Dec;20(4):340-346. 10.4048/jbc.2017.20.4.340.
A Multicenter Phase II Trial of Neoadjuvant Chemotherapy with Docetaxel and Gemcitabine in Locally Advanced Breast Cancer
- Affiliations
-
- 1Department of Surgery, St. Vincent's Hospital, College of Medicine, The Catholic University of Korea, Suwon, Korea. yjsuh@catholic.ac.kr
- 2Department of Surgery, Inje University Busan Paik Hospital, Busan, Korea.
- 3Department of Surgery, Chonbuk National University Hospital, Jeonju, Korea.
- 4Department of Surgery, Ajou University School of Medicine, Suwon, Korea.
- 5Department of Surgery, Inje University Sanggye Paik Hospital, Seoul, Korea.
- 6Department of Surgery, Presbyterian Medical Center, Jeonju, Korea.
- 7Department of Surgery, Uijeongbu St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Uijeongbu, Korea.
- KMID: 2398203
- DOI: http://doi.org/10.4048/jbc.2017.20.4.340
Abstract
- PURPOSE
The current multicenter phase II study was conducted to evaluate the efficacy and safety of the combination of docetaxel and gemcitabine as neoadjuvant chemotherapy (NAC) for locally advanced breast cancer.
METHODS
A total of 98 patients with stage II-III breast cancer were enrolled. The primary endpoint was pathological complete response (pCR) rate of invasive cancer after the completion of the fourth cycle of NAC. The secondary endpoints included response rate (RR), rate of breast-conserving surgery, toxicity, and disease-free survival (DFS). This study is registered with ClinicalTrials.gov (NCT01352494).
RESULTS
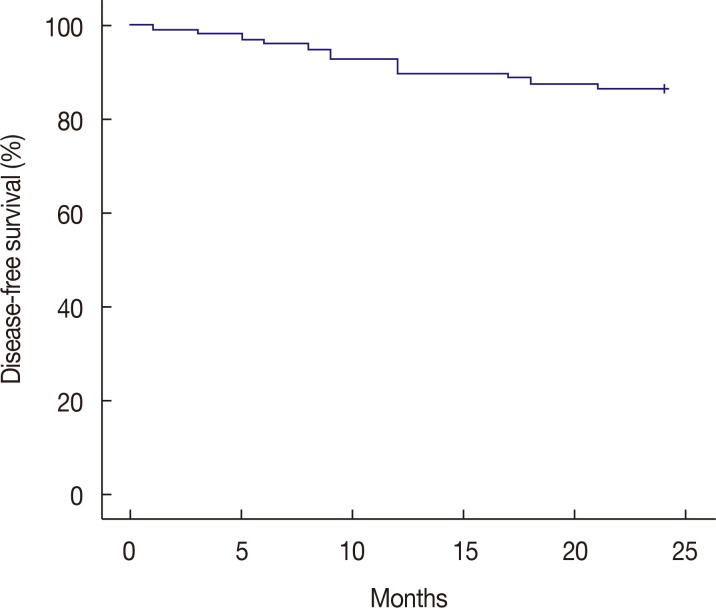
pCR in the breast and the axillary lymph node was observed in seven of the 98 enrolled patients (7.1%). The overall clinical RR, including partial responses, was 65.3%. Breast-conserving surgery was performed in 75 of the 98 assessable patients (76.5%). Neutropenia was frequent and was observed in 92 of the 98 patients (93.9%), including grade 3 and 4 in 24 patients (24.5%) and 63 patients (64.3%), respectively. Dose reductions were required for 30 of the 92 patients (32.6%). After a median follow-up of 24 months, the overall DFS of the group was 86.7%.
CONCLUSION
The combination of docetaxel and gemcitabine did not improve pCR. However, this regimen has shown potential as a NAC by producing a reasonable rate of breast-conserving surgery and favorable responses in patients with locally advanced breast cancer. The therapeutic efficacy of this regimen will be determined in additional trials to overcome the limitations of the current study.
Keyword
MeSH Terms
Figure
Reference
-
1. Kaufmann M, Hortobagyi GN, Goldhirsch A, Scholl S, Makris A, Valagussa P, et al. Recommendations from an international expert panel on the use of neoadjuvant (primary) systemic treatment of operable breast cancer: an update. J Clin Oncol. 2006; 24:1940–1949. PMID: 16622270.
Article2. von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012; 30:1796–1804. PMID: 22508812.
Article3. van der Hage JA, van de Velde CJ, Julien JP, Tubiana-Hulin M, Vandervelden C, Duchateau L. Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. J Clin Oncol. 2001; 19:4224–4237. PMID: 11709566.
Article4. Vriens BE, Aarts MJ, de Vries B, van Gastel SM, Wals J, Smilde TJ, et al. Doxorubicin/cyclophosphamide with concurrent versus sequential docetaxel as neoadjuvant treatment in patients with breast cancer. Eur J Cancer. 2013; 49:3102–3110. PMID: 23850450.
Article5. Kuerer HM, Newman LA, Smith TL, Ames FC, Hunt KK, Dhingra K, et al. Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J Clin Oncol. 1999; 17:460–469. PMID: 10080586.
Article6. Mavroudis D, Malamos N, Alexopoulos A, Kourousis C, Agelaki S, Sarra E, et al. Salvage chemotherapy in anthracycline-pretreated metastatic breast cancer patients with docetaxel and gemcitabine: a multicenter phase II trial. Ann Oncol. 1999; 10:211–215. PMID: 10093691.
Article7. O'Shaughnessy J, Miles D, Vukelja S, Moiseyenko V, Ayoub JP, Cervantes G, et al. Superior survival with capecitabine plus docetaxel combination therapy in anthracycline-pretreated patients with advanced breast cancer: phase III trial results. J Clin Oncol. 2002; 20:2812–2823. PMID: 12065558.8. Kelly CM, Green MC, Broglio K, Thomas ES, Brewster AM, Valero V, et al. Phase III trial evaluating weekly paclitaxel versus docetaxel in combination with capecitabine in operable breast cancer. J Clin Oncol. 2012; 30:930–935. PMID: 22331946.
Article9. Del Mastro L, Fabi A, Mansutti M, De Laurentiis M, Durando A, Colantuoni G, et al. Phase III trial of first-line treatment with gemcitabine plus docetaxel versus gemcitabine plus paclitaxel in women with metastatic breast cancer (MBC): a comparison of different schedules and treatment. In : 2012 ASCO Annual Meeting; 2012. p. 30. Abstract #1073.10. Tomova A, Bartsch R, Brodowicz T, Tzekova V, Timcheva C, Wiltschke C, et al. Concomitant docetaxel plus gemcitabine versus sequential docetaxel followed by gemcitabine in anthracycline-pretreated metastatic or locally recurrent inoperable breast cancer patients: a prospective multicentre trial of the Central European Cooperative Oncology Group (CECOG). Breast Cancer Res Treat. 2010; 119:169–176. PMID: 19768533.
Article11. Heinemann V. Role of gemcitabine in the treatment of advanced and metastatic breast cancer. Oncology. 2003; 64:191–206. PMID: 12697958.
Article12. Joensuu H, Sailas L, Alanko T, Sunela K, Huuhtanen R, Utriainen M, et al. Docetaxel versus docetaxel alternating with gemcitabine as treatments of advanced breast cancer: final analysis of a randomised trial. Ann Oncol. 2010; 21:968–973. PMID: 19819914.
Article13. Estévez LG, Sánchez-Rovira P, Dómine M, León A, Calvo I, Jaén A, et al. Biweekly docetaxel and gemcitabine as neoadjuvant chemotherapy followed by adjuvant doxorubicin and cyclophosphamide therapy in stage II and III breast cancer patients: results of a phase II study. Clin Transl Oncol. 2007; 9:317–322. PMID: 17525042.
Article14. Yardley DA. Gemcitabine and docetaxel in metastatic and neoadjuvant treatment of breast cancer. Semin Oncol. 2004; 31(2 Suppl 5):37–44.
Article15. Diéras V, Fumoleau P, Romieu G, Tubiana-Hulin M, Namer M, Mauriac L, et al. Randomized parallel study of doxorubicin plus paclitaxel and doxorubicin plus cyclophosphamide as neoadjuvant treatment of patients with breast cancer. J Clin Oncol. 2004; 22:4958–4965. PMID: 15611510.
Article16. Buzdar AU. Preoperative chemotherapy treatment of breast cancer: a review. Cancer. 2007; 110:2394–2407. PMID: 17941030.17. Smorenburg CH, Bontenbal M, Seynaeve C, van Zuylen C, de Heus G, Verweij J, et al. Phase II study of weekly gemcitabine in patients with metastatic breast cancer relapsing or failing both an anthracycline and a taxane. Breast Cancer Res Treat. 2001; 66:83–87. PMID: 11368414.
Article18. Rha SY, Moon YH, Jeung HC, Kim YT, Sohn JH, Yang WI, et al. Gemcitabine monotherapy as salvage chemotherapy in heavily pretreated metastatic breast cancer. Breast Cancer Res Treat. 2005; 90:215–221. PMID: 15830134.
Article19. Modi S, Currie VE, Seidman AD, Bach AM, Panageas KS, Theodoulou M, et al. A phase II trial of gemcitabine in patients with metastatic breast cancer previously treated with an anthracycline and taxane. Clin Breast Cancer. 2005; 6:55–60. PMID: 15899073.
Article20. Metro G, Fabi A, Russillo M, Papaldo P, De Laurentiis M, Ferretti G, et al. Taxanes and gemcitabine doublets in the management of HER-2 negative metastatic breast cancer: towards optimization of association and schedule. Anticancer Res. 2008; 28:1245–1258. PMID: 18505062.21. Yang Y, Im SA, Keam B, Lee KH, Kim TY, Suh KJ, et al. Prognostic impact of AJCC response criteria for neoadjuvant chemotherapy in stage II/III breast cancer patients: breast cancer subtype analyses. BMC Cancer. 2016; 16:515. PMID: 27444430.
Article22. McCarthy N, Boyle F, Zdenkowski N, Bull J, Leong E, Simpson A, et al. Neoadjuvant chemotherapy with sequential anthracycline-docetaxel with gemcitabine for large operable or locally advanced breast cancer: ANZ 0502 (NeoGem). Breast. 2014; 23:142–151. PMID: 24393617.
Article23. von Minckwitz G, Kümmel S, du Bois A, Eiermann W, Eidtmann H, Gerber B, et al. Pegfilgrastim +/- ciprofloxacin for primary prophylaxis with TAC (docetaxel/doxorubicin/cyclophosphamide) chemotherapy for breast cancer: results from the GEPARTRIO study. Ann Oncol. 2008; 19:292–298. PMID: 17846019.
Article24. Von Minckwitz G, Loibl S, Untch M. What is the current standard of care for anti-HER2 neoadjuvant therapy in breast cancer? Oncology (Williston Park). 2012; 26:20–26. PMID: 22393792.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Neoadjuvant Chemotherapy with Docetaxel and Adriamycin in Breast Cancer; Clincopathologic Factors Influencing to Response Rate
- Clinical outcome and predictive factors for docetaxel and epirubicin neoadjuvant chemotherapy of locally advanced breast cancer
- Curative Resection of Inoperable, Locally Advanced Gastric Cancer after Neoadjuvant Chemotherapy with Taxotere and Cisplatin
- Short Term Effect of Neoadjuvant Therapy with Docetaxel and Adriamycin in Advanced Breast Cancer
- Preoperative Chemotherapy in Advanced Stomach Cancer (Pros)