J Korean Ophthalmol Soc.
2017 Dec;58(12):1376-1387. 10.3341/jkos.2017.58.12.1376.
Changes of the Individual Retinal Layer Thickness in Non-proliferative Diabetic Retinopathy in Type 2 Diabetes
- Affiliations
-
- 1Department of Ophthalmology, Wonkwang University School of Medicine, Iksan, Korea. cuchoi77@hanmail.net
- 2Institute of Wonkwang Medical Science, Wonkwang University, Iksan, Korea.
- KMID: 2397852
- DOI: http://doi.org/10.3341/jkos.2017.58.12.1376
Abstract
- PURPOSE
To compare retinal layer thickness in non-proliferative diabetic retinopathy in type 2 diabetic patients as measured by optical coherence tomography.
METHODS
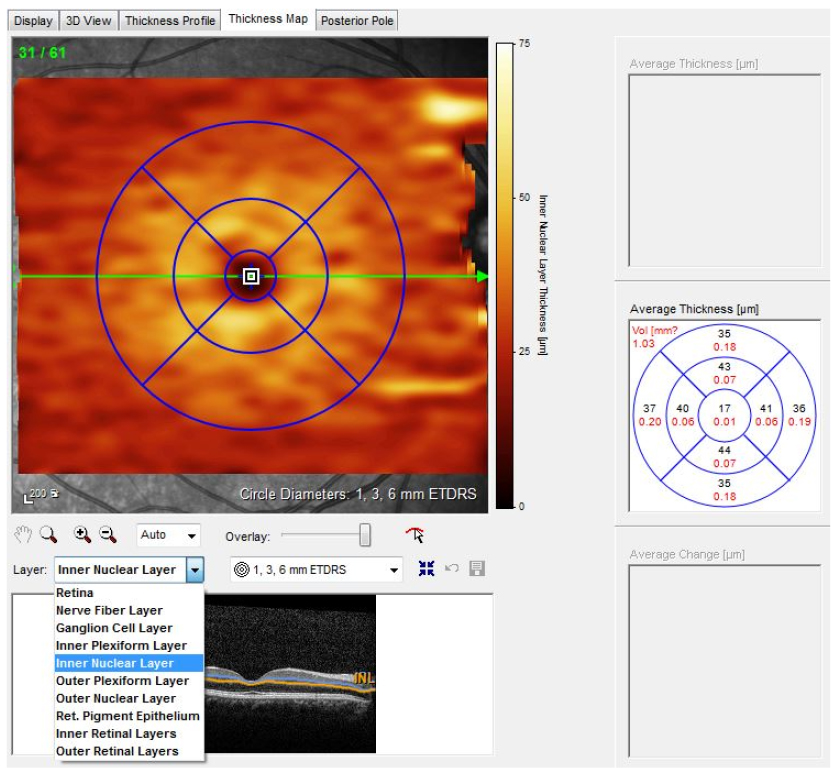
A total of 108 eyes from 71 patients, between January 2015 and July 2016, were included in this study. Of these, 39 eyes were included in the control group, 38 eyes in the diabetic group without non-proliferative diabetic retinopathy, and 31 eyes in the non-proliferative diabetic retinopathy group (NPDR). We measured the thickness of each retinal layer by optical coherence tomography (OCT). A total of ten layers were evaluated including the retinal nerve fiber layer (RNFL), ganglion cell layer (GCL), inner plexiform layer (IPL), inner nuclear layer (INL), outer plexiform layer (OPL), outer nuclear layer (ONL), retinal pigment epithelium (RPE), inner retinal layer (IRL), outer retinal layer (ORL), and the total retinal layer (TRL). We compared the superior, inferior, nasal, and temporal regions at 1-3mm from the central fovea.
RESULTS
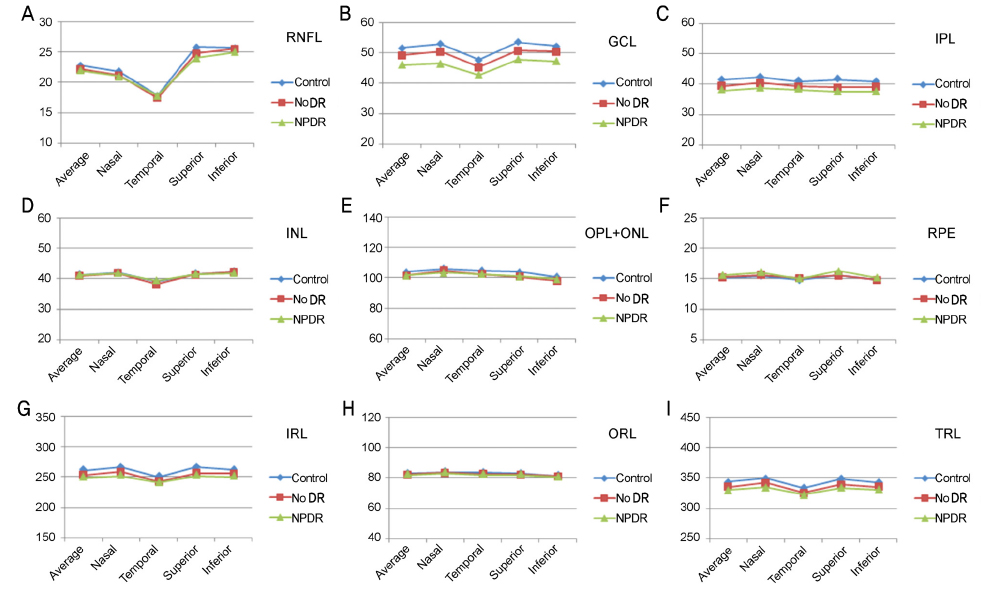
RNFL was thinner in the superior region of the NPDR, as compared with that of the control group, showing statistical significance (p = 0.016). The thickness of all regions in the GCL, IPL, and IRL were decreased in NPDR, as compared to the control group with statistical significance. In addition, the thickness of the superior region in the GCL, IPL, and IRL showed statistically significant differences between controls and the no diabetic retinopathy (DR) group (p = 0.026, 0.003, 0.003). The thickness of the INL, OPL plus ONL, RPE, and ORL in all three groups showed no significant difference. The differences in the decreased thickness in the IRL were similar to that of TRL.
CONCLUSIONS
Retinal neurodegeneration was observed in the IRL, which included changes to the RNFL, GCL, and IPL in early type 2 diabetes before microvascular injury was apparent. Thorough control of blood glucose is required in early diabetes, and further studies to delay retinal neurodegeneration are required. OCT might have an important role in early diagnosis and follow up of diabetic retinopathy.
Keyword
MeSH Terms
Figure
Reference
-
1. Group ETDRSR. Grading diabetic retinopathy from stereoscopic color fundus photographs--an extension of the modified Airlie House classification. ETDRS report number 10. Ophthalmology. 1991; 98:5 Suppl. 786–806.2. Cogan DG, Toussaint D, Kuwabara T. Retinal vascular patterns. IV. Diabetic retinopathy. Arch Ophthalmol. 1961; 66:366–378.3. Gastinger MJ, Singh RS, Barber AJ. Loss of cholinergic and dopaminergic amacrine cells in streptozotocin-diabetic rat and Ins2Akita-diabetic mouse retinas. Invest Ophthalmol Vis Sci. 2006; 47:3143–3150.4. Ly A, Yee P, Vessey KA, et al. Early inner retinal astrocyte dysfunction during diabetes and development of hypoxia, retinal stress, and neuronal functional loss. Invest Ophthalmol Vis Sci. 2011; 52:9316–9326.5. Barber AJ, Gardner TW, Abcouwer SF. The significance of vascular and neural apoptosis to the pathology of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2011; 52:1156–1163.6. Fletcher EL, Phipps JA, Wilkinson-Berka JL. Dysfunction of retinal neurons and glia during diabetes. Clin Exp Optom. 2005; 88:132–145.7. Villarroel M, Ciudin A, Hernández C, Simó R. Neurodegeneration: an early event of diabetic retinopathy. World J Diabetes. 2010; 1:57–64.8. Chhablani J, Rao HB, Begum VU, et al. Retinal ganglion cells thinning in eyes with nonproliferative idiopathic macular telangiectasia type 2A. Invest Ophthalmol Vis Sci. 2015; 56:1416–1422.9. Araie M, Saito H, Tomidokoro A, et al. Relationship between macular inner retinal layer thickness and corresponding retinal sensitivity in normal eyes inner retinal layer thickness effect on sensitivity. Invest Ophthalmol Vis Sci. 2014; 55:7199–7205.10. Sabater AL, Velázquez-Villoria Á, Zapata MA, et al. Evaluation of macular retinal ganglion cell-inner plexiform layer thickness after vitrectomy with internal limiting membrane peeling for idiopathic macular holes. Biomed Res Int. 2014; 2014:458631.11. Yang JY, Kim NK, Lee YJ, et al. Prevalence and factors associated with diabetic retinopathy in a Korean adult population: the 2008–2009 Korea National Health and Nutrition Examination Survey. Diabetes Res Clin Pract. 2013; 102:218–224.12. Park CY, Park SE, Bae JC, et al. Prevalence of and risk factors for diabetic retinopathy in Koreans with type II diabetes: baseline characteristics of Seoul Metropolitan City-Diabetes Prevention Program (SMC-DPP) participants. Br J Ophthalmol. 2012; 96:151–155.13. Qin Y, Xu G, Wang W. Dendritic abnormalities in retinal ganglion cells of three-month diabetic rats. Curr Eye Res. 2006; 31:967–974.14. Kadłubowska J, Malaguarnera L, Wąż P, Zorena K. Neurodegeneration and neuroinflammation in diabetic retinopathy: potential approaches to delay neuronal loss. Curr Neuropharmacol. 2016; 14:831–839.15. van Dijk HW, Verbraak FD, Stehouwer M, et al. Association of visual function and ganglion cell layer thickness in patients with diabetes mellitus type 1 and no or minimal diabetic retinopathy. Vision Res. 2011; 51:224–228.16. Vujosevic S, Micera A, Bini S, et al. Aqueous humor biomarkers of Müller cell activation in diabetic eyesMüller cell activation in diabetics. Invest Ophthalmol Vis Sci. 2015; 56:3913–3918.17. Matteucci A, Gaddini L, Villa M, et al. Neuroprotection by rat Müller glia against high glucose-induced neurodegeneration through a mechanism involving ERK1/2 activation. Exp Eye Res. 2014; 125:20–29.18. Faghihi H, Hajizadeh F, Hashemi H, Khabazkhoob M. Agreement of two different spectral domain optical coherence tomography instruments for retinal nerve fiber layer measurements. J Ophthalmic Vis Res. 2014; 9:31–37.19. Liu X, Shen M, Huang S, et al. Repeatability and reproducibility of eight macular intra-retinal layer thicknesses determined by an automated segmentation algorithm using two SD-OCT instruments. PLoS One. 2014; 9:e87996.20. Lee HJ, Kim MS, Jo YJ, Kim JY. Ganglion cell–inner plexiform layer thickness in retinal diseases: Repeatability study of spectral-domain optical coherence tomography. Am J Ophthalmol. 2015; 160:283–289.e1.21. Chen Y, Li J, Yan Y, Shen X. Diabetic macular morphology changes may occur in the early stage of diabetes. BMC Ophthalmol. 2016; 16:12.22. Appukuttan B, Giridhar A, Gopalakrishnan M, Sivaprasad S. Normative spectral domain optical coherence tomography data on macular and retinal nerve fiber layer thickness in Indians. Indian J Ophthalmol. 2014; 62:316–321.23. Kang JH, Kim SA, Song WG, Yoon HS. Macular thickness changes with age in normal subjects measured by optical coherence tomography. J Korean Ophthalmol Soc. 2004; 45:592–598.24. Kim CH, Jin SY, Lee YH, Chang YS. Analysis of macular layer thickness measured using spectral domain optical coherence tomography in Korean subjects. J Korean Ophthalmol Soc. 2016; 57:264–275.25. Jonas JB, Naumann GO. Parapapillary retinal vessel diameter in normal and glaucoma eyes. II. Correlations. Invest Ophthalmol Vis Sci. 1989; 30:1604–1611.26. Kern TS, Engerman RL. Vascular lesions in diabetes are distributed non-uniformly within the retina. Exp Eye Res. 1995; 60:545–549.27. Anand-Apte B, Hollyfield JG. Developmental anatomy of the retinal and choroidal vasculature. Cleveland: Elsevier Ltd;2010. p. 9–15.28. Savastano MC, Lumbroso B, Rispoli M. In vivo characterization of retinal vascularization morphology using optical coherence tomography angiography. Retina. 2015; 35:2196–2203.29. Couturier A, Mané V, Bonnin S, et al. Capillary plexus anomalies in diabetic retinopathy on optical coherence tomography angiography. Retina. 2015; 35:2384–2391.30. Talisa E, Chin AT, Bonini Filho MA, et al. Detection of microvascular changes in eyes of patients with diabetes but not clinical diabetic retinopathy using optical coherence tomography angiography. Retina. 2015; 35:2364–2370.31. Garcia-Ramírez M, Hernández C, Villarroel M, et al. Interphotoreceptor retinoid-binding protein (IRBP) is downregulated at early stages of diabetic retinopathy. Diabetologia. 2009; 52:2633–2641.32. Valverde AM, Miranda S, García-Ramírez M, et al. Proapoptotic and survival signaling in the neuroretina at early stages of diabetic retinopathy. Mol Vis. 2013; 19:47–53.33. Simó R, Sundstrom JM, Antonetti DA. Ocular anti-VEGF therapy for diabetic retinopathy: the role of VEGF in the pathogenesis of diabetic retinopathy. Diabetes Care. 2014; 37:893–899.34. Kern TS, Barber AJ. Retinal ganglion cells in diabetes. J Physiol. 2008; 586:4401–4408.35. Padhy D, Rao A. Macular ganglion cell/inner plexiform layer measurements by spectral domain optical coherence tomography for detection of early glaucoma and comparison to retinal nerve fiber layer measurements. Am J Ophthalmol. 2014; 158:211.36. Abu El-Asrar AM, Dralands L, Missotten L, Geboes K. Expression of antiapoptotic and proapoptotic molecules in diabetic retinas. Eye (Lond). 2007; 21:238–245.37. Abu-El-Asrar AM, Dralands L, Missotten L, et al. Expression of apoptosis markers in the retinas of human subjects with diabetes. Invest Ophthalmol Vis Sci. 2004; 45:2760–2766.38. Carpineto P, Toto L, Aloia R, et al. Neuroretinal alterations in the early stages of diabetic retinopathy in patients with type 2 diabetes mellitus. Eye (Lond). 2016; 30:673–679.39. Barber AJ, Nakamura M, Wolpert EB, et al. Insulin rescues retinal neurons from apoptosis by a phosphatidylinositol 3-kinase/Akt-mediated mechanism that reduces the activation of caspase-3. J Biol Chem. 2001; 276:32814–32821.40. Fine BS, Brucker AJ. Macular edema and cystoid macular edema. Am J Ophthalmol. 1981; 92:466–481.41. Murakami T, Nishijima K, Akagi T, et al. Optical coherence tomographic reflectivity of photoreceptors beneath cystoid spaces in diabetic macular edema. Invest Ophthalmol Vis Sci. 2012; 53:1506–1511.42. Bandello F, Tejerina AN, Vujosevic S, et al. Retinal layer location of increased retinal thickness in eyes with subclinical and clinical macular edema in diabetes type 2. Ophthalmic Res. 2015; 54:112–117.43. Picconi F, Parravano M, Ylli D, et al. Retinal neurodegeneration in patients with type 1 diabetes mellitus: the role of glycemic variability. Acta Diabetol. 2017; 54:489–497.44. Matsumoto H, Sato T, Kishi S. Outer nuclear layer thickness at the fovea determines visual outcomes in resolved central serous chorioretinopathy. Am J Ophthalmol. 2009; 148:105–110.e1.45. Curcio CA, Messinger JD, Sloan KR, et al. Human chorioretinal layer thicknesses measured in macula-wide, high-resolution histologic sections. Invest Ophthalmol Vis Sci. 2011; 52:3943–3954.46. Otani T, Yamaguchi Y, Kishi S. Improved visualization of Henle fiber layer by changing the measurement beam angle on optical coherence tomography. Retina. 2011; 31:497–501.47. Lujan BJ, Roorda A, Knighton RW, Carroll J. Revealing Henle's fiber layer using spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2011; 52:1486–1492.48. Ouyang Y, Walsh AC, Keane PA, et al. Different phenotypes of the appearance of the outer plexiform layer on optical coherence tomography. Graefes Arch Clin Exp Ophthalmol. 2013; 251:2311–2317.49. Sohn EH, van Dijk HW, Jiao C, et al. Retinal neurodegeneration may precede microvascular changes characteristic of diabetic retinopathy in diabetes mellitus. Proc Natl Acad Sci U S A. 2016; 113:E2655–E2664.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Contrast Sensitivity and Inner Retinal Layer Thickness Analysis of Type 2 Diabetic Patients Without Retinopathy
- Difference of GCIPL Thickness of Diabetes and Normal Eyes in Spectral Domain OCT
- Analysis of the Optic Nerve Head and RNFL Thickness Using Optical Coherence Tomography in Diabetes
- Clinical Analysis of Diabetic Retinopathy According to the Type of Diabetes Mellitus
- High Serum Lipoprotein ( a ) Levels in Korean Type 2 Diabetic Patients with Proliferative Diabetic retinopathy