Asia Pac Allergy.
2011 Oct;1(3):157-167. 10.5415/apallergy.2011.1.3.157.
Overview on the pathomechanisms of allergic rhinitis
- Affiliations
-
- 1Nippon Medical School, Tokyo 113-8603, Japan. pawankar.ruby@gmail.com
- KMID: 2397435
- DOI: http://doi.org/10.5415/apallergy.2011.1.3.157
Abstract
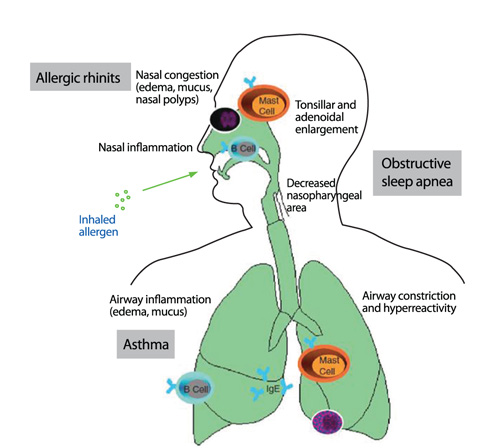
- Allergic rhinitis a chronic inflammatory disease of the upper airways that has a major impact on the quality of life of patients and is a socio-economic burden. Understanding the underlying immune mechanisms is central to developing better and more targeted therapies. The inflammatory response in the nasal mucosa includes an immediate IgE-mediated mast cell response as well as a latephase response characterized by recruitment of eosinophils, basophils, and T cells expressing Th2 cytokines including interleukin (IL)-4, a switch factor for IgE synthesis, and IL-5, an eosinophil growth factor and on-going allergic inflammation. Recent advances have suggested new pathways like local synthesis of IgE, the IgE-IgE receptor mast cell cascade in on-going allergic inflammation and the epithelial expression of cytokines that regulate Th2 cytokine responses (i.e., thymic stromal lymphopoietin, IL-25, and IL-33). In this review, we briefly review the conventional pathways in the pathophysiology of allergic rhinitis and then elaborate on the recent advances in the pathophysiology of allergic rhinitis. An improved understanding of the immune mechanisms of allergic rhinitis can provide a better insight on novel therapeutic targets.
Keyword
MeSH Terms
Figure
Cited by 4 articles
-
Current trends in upper airways and ocular allergic inflammation
Ashok Shah
Asia Pac Allergy. 2011;1(3):105-107. doi: 10.5415/apallergy.2011.1.3.105.Asia Pacific allergy: four years of experience
Yoon-Seok Chang
Asia Pac Allergy. 2015;5(1):1-2. doi: 10.5415/apallergy.2015.5.1.1.Asia Pacific Allergy: it's been five years!
Yoon-Seok Chang
Asia Pac Allergy. 2016;6(1):1-2. doi: 10.5415/apallergy.2016.6.1.1.Influence of the Genetic Background on Allergic Rhinitis Models in Mice
Roza Khalmuratova, Hyun-Woo Shin
Clin Exp Otorhinolaryngol. 2020;13(4):322-323. doi: 10.21053/ceo.2020.00892.
Reference
-
1. Strachan D, Sibbald B, Weiland S, Aït-Khaled N, Anabwani G, Anderson HR, Asher MI, Beasley R, Björkstén B, Burr M, Clayton T, Crane J, Ellwood P, Keil U, Lai C, Mallol J, Martinez F, Mitchell E, Montefort S, Pearce N, Robertson C, Shah J, Stewart A, von Mutius E, Williams H. Worldwide variations in prevalence of symptoms of allergic rhinoconjunctivitis in children: the International Study of Asthma and Allergies in Childhood (ISAAC). Pediatr Allergy Immunol. 1997. 8:161–176.
Article2. Simons FE. Learning impairment and allergic rhinitis. Allergy Asthma Proc. 1996. 17:185–189.
Article3. Cockburn IM, Bailit HL, Berndt ER, Finkelstein SN. Loss of work productivity due to illness and medical treatment. J Occup Environ Med. 1999. 41:948–953.
Article4. Bousquet J, Bullinger M, Fayol C, Marquis P, Valentin B, Burtin B. Assessment of quality of life in patients with perennial allergic rhinitis with the French version of the SF-36 Health Status Questionnaire. J Allergy Clin Immunol. 1994. 94:182–188.
Article5. Malone DC, Lawson KA, Smith DH, Arrighi HM, Battista C. A cost of illness study of allergic rhinitis in the United States. J Allergy Clin Immunol. 1997. 99:22–27.
Article6. Pawankar R, Bunnag C, Chen Y, Fukuda T, Kim YY, Le LT, Huong le TT, O'Hehir RE, Ohta K, Vichyanond P, Wang DY, Zhong N, Khaltaev N, Bousquet J. Allergic rhinitis and its impact on asthma update (ARIA 2008)--western and Asian-Pacific perspective. Asian Pac J Allergy Immunol. 2009. 27:237–243.7. Naclerio RM. Allergic rhinitis. N Engl J Med. 1991. 325:860–869.
Article8. Bousquet J, Van Cauwenberge P, Khaltaev N. Allergic rhinitis and its impact on asthma. J Allergy Clin Immunol. 2001. 108:S147–S334.
Article9. Young MC. Rhinitis, sinusitis, and polyposis. Allergy Asthma Proc. 1998. 19:211–218.
Article10. North ML, Ellis AK. The role of epigenetics in the developmental origins of allergic disease. Ann Allergy Asthma Immunol. 2011. 106:355–361.
Article11. Hollingsworth JW, Maruoka S, Boon K, Garantziotis S, Li Z, Tomfohr J, Bailey N, Potts EN, Whitehead G, Brass DM, Schwartz DA. In utero supplementation with methyl donors enhances allergic airway disease in mice. J Clin Invest. 2008. 118:3462–3469.
Article12. Bradding P, Feather IH, Wilson S, Bardin PG, Heusser CH, Holgate ST, Howarth PH. Immunolocalization of cytokines in the nasal mucosa of normal and perennial rhinitic subjects. The mast cell as a source of IL-4, IL-5, and IL-6 in human allergic mucosal inflammation. J Immunol. 1993. 151:3853–3865.13. Pawankar RU, Okuda M, Hasegawa S, Suzuki K, Yssel H, Okubo K, Okumura K, Ra C. Interleukin-13 expression in the nasal mucosa of perennial allergic rhinitis. Am J Respir Crit Care Med. 1995. 152:2059–2067.
Article14. Pawankar R, Ra C. Heterogeneity of mast cells and T cells in the nasal mucosa. J Allergy Clin Immunol. 1996. 98:S248–S262.
Article15. Ozu C, Pawankar R, Takizawa R, Yamagishi S, Yagi T. Regulation of mast cell migration into the allergic nasal epithelium by RANTES and not SCF. J Allergy Clin Immunol. 2004. 113:S28.16. Lilly CM, Nakamura H, Kesselman H, Nagler-Anderson C, Asano K, Garcia-Zepeda EA, Rothenberg ME, Drazen JM, Luster AD. Expression of eotaxin by human lung epithelial cells: induction by cytokines and inhibition by glucocorticoids. J Clin Invest. 1997. 99:1767–1773.
Article17. Li L, Xia Y, Nguyen A, Lai YH, Feng L, Mosmann TR, Lo D. Effects of Th2 cytokines on chemokine expression in the lung: IL-13 potently induces eotaxin expression by airway epithelial cells. J Immunol. 1999. 162:2477–2487.18. Sekiya T, Miyamasu M, Imanishi M, Yamada H, Nakajima T, Yamaguchi M, Fujisawa T, Pawankar R, Sano Y, Ohta K, Ishii A, Morita Y, Yamamoto K, Matsushima K, Yoshie O, Hirai K. Inducible expression of a Th2-type CC chemokine thymus- and activation-regulated chemokine by human bronchial epithelial cells. J Immunol. 2000. 165:2205–2213.
Article19. Pawankar R. Mast cells as orchestrators of the allergic reaction: the IgE-IgE receptor mast cell network. Curr Opin Allergy Clin Immunol. 2001. 1:3–6.
Article20. Irani AA, Schechter NM, Craig SS, DeBlois G, Schwartz LB. Two types of human mast cells that have distinct neutral protease compositions. Proc Natl Acad Sci U S A. 1986. 83:4464–4468.
Article21. Enerbäck L, Pipkorn U, Olofsson A. Intraepithelial migration of mucosal mast cells in hay fever: ultrastructural observations. Int Arch Allergy Appl Immunol. 1986. 81:289–297.
Article22. Nilsson G, Hjertson M, Andersson M, Greiff L, Svensson C, Nilsson K, Siegbahn A. Demonstration of mast-cell chemotactic activity in nasal lavage fluid: characterization of one chemotaxin as c-kit ligand, stem cell factor. Allergy. 1998. 53:874–879.
Article23. Salib RJ, Kumar S, Wilson SJ, Howarth PH. Nasal mucosal immunoexpression of the mast cell chemoattractants TGF-beta, eotaxin, and stem cell factor and their receptors in allergic rhinitis. J Allergy Clin Immunol. 2004. 114:799–806.24. Pawankar R, Yamagishi S, Takizawa R, Yagi T. Mast cell-IgE-and mast cell-structural cell interactions in allergic airway disease. Curr Drug Targets Inflamm Allergy. 2003. 2:303–312.
Article25. Toru H, Pawankar R, Ra C, Yata J, Nakahata T. Human mast cells produce IL-13 by high-affinity IgE receptor cross-linking: enhanced IL-13 production by IL-4-primed human mast cells. J Allergy Clin Immunol. 1998. 102:491–502.
Article26. Pawankar R, Okuda M, Yssel H, Okumura K, Ra C. Nasal mast cells in perennial allergic rhinitics exhibit increased expression of the Fc epsilonRI, CD40L, IL-4, and IL-13, and can induce IgE synthesis in B cells. J Clin Invest. 1997. 99:1492–1499.
Article27. Pawankar R, Ra C. IgE-Fc epsilonRI-mast cell axis in the allergic cycle. Clin Exp Allergy. 1998. 28:Suppl 3. 6–14.28. Naclerio RM, Proud D, Togias AG, Adkinson NF Jr, Meyers DA, Kagey-Sobotka A, Plaut M, Norman PS, Lichtenstein LM. Inflammatory mediators in late antigen-induced rhinitis. N Engl J Med. 1985. 313:65–70.
Article29. Silberstein DS. Eosinophil function in health and disease. Crit Rev Oncol Hematol. 1995. 19:47–77.
Article30. Denburg JA. Bone marrow in atopy and asthma: hematopoietic mechanisms in allergic inflammation. Immunol Today. 1999. 20:111–113.
Article31. Alam R, Stafford S, Forsythe P, Harrison R, Faubion D, Lett-Brown MA, Grant JA. RANTES is a chemotactic and activating factor for human eosinophils. J Immunol. 1993. 150:3442–3448.32. Baggiolini M, Dahinden CA. CC chemokines in allergic inflammation. Immunol Today. 1994. 15:127–133.
Article33. Garcia-Zepeda EA, Rothenberg ME, Ownbey RT, Celestin J, Leder P, Luster AD. Human eotaxin is a specific chemoattractant for eosinophil cells and provides a new mechanism to explain tissue eosinophilia. Nat Med. 1996. 2:449–456.
Article34. Simon HU, Yousefi S, Schranz C, Schapowal A, Bachert C, Blaser K. Direct demonstration of delayed eosinophil apoptosis as a mechanism causing tissue eosinophilia. J Immunol. 1997. 158:3902–3908.35. Simon HU. Eosinophil apoptosis in allergic diseases--an emerging new issue. Clin Exp Allergy. 1998. 28:1321–1324.36. Plager DA, Stuart S, Gleich GJ. Human eosinophil granule major basic protein and its novel homolog. Allergy. 1998. 53:33–40.
Article37. Venge P, Byström J, Carlson M, Hâkansson L, Karawacjzyk M, Peterson C, Sevéus L, Trulson A. Eosinophil cationic protein (ECP): molecular and biological properties and the use of ECP as a marker of eosinophil activation in disease. Clin Exp Allergy. 1999. 29:1172–1186.
Article38. Rosenberg HF. The eosinophil ribonucleases. Cell Mol Life Sci. 1998. 54:795–803.
Article39. Egesten A, Weller PF, Olsson I. Arylsulfatase B is present in crystalloid-containing granules of human eosinophil granulocytes. Int Arch Allergy Immunol. 1994. 104:207–210.
Article40. Broide DH, Paine MM, Firestein GS. Eosinophils express interleukin 5 and granulocyte macrophage-colony-stimulating factor mRNA at sites of allergic inflammation in asthmatics. J Clin Invest. 1992. 90:1414–1424.
Article41. KleinJan A, Dijkstra MD, Boks SS, Severijnen LA, Mulder PG, Fokkens WJ. Increase in IL-8, IL-10, IL-13, and RANTES mRNA levels (in situ hybridization) in the nasal mucosa after nasal allergen provocation. J Allergy Clin Immunol. 1999. 103:441–450.
Article42. Yang PC, Okuda M, Pawankar R, Aihara K. Electron microscopical studies of the cell population in nasal secretions. Rhinology. 1995. 33:70–77.43. Wang D, Clement P, Smitz J, De Waele M, Derde MP. Correlations between complaints, inflammatory cells and mediator concentrations in nasal secretions after nasal allergen challenge and during natural allergen exposure. Int Arch Allergy Immunol. 1995. 106:278–285.
Article44. Arzuaga Orozco J, Segura Méndez NH, Martínez Cairo-Cueto S. Evaluation of eosinophils in nasal mucus from patients with perennial allergic rhinitis during nasal provocation tests. Rev Alerg Mex. 1993. 40:139–141.45. Pawankar R, Yamagishi S, Nonaka M, Koichi H, Ozu C, Watanabe S. Synergistic Induction of TARC in nasal epithelial cells and fibroblasts by IL-4 IL-13 and TNF-alpha and its correlation to CCR4+T cells in patients with allergic rhinitis. J Allergy Clin Immunol. 2003. 111:S148.46. Varney VA, Jacobson MR, Sudderick RM, Robinson DS, Irani AM, Schwartz LB, Mackay IS, Kay AB, Durham SR. Immunohistology of the nasal mucosa following allergen-induced rhinitis. Identification of activated T lymphocytes, eosinophils, and neutrophils. Am Rev Respir Dis. 1992. 146:170–176.
Article47. Pawankar RU, Okuda M, Okubo K, Ra C. Lymphocyte subsets of the nasal mucosa in perennial allergic rhinitis. Am J Respir Crit Care Med. 1995. 152:2049–2058.
Article48. Durham SR, Ying S, Varney VA, Jacobson MR, Sudderick RM, Mackay IS, Kay AB, Hamid QA. Cytokine messenger RNA expression for IL-3, IL-4, IL-5, and granulocyte/macrophage-colony-stimulating factor in the nasal mucosa after local allergen provocation: relationship to tissue eosinophilia. J Immunol. 1992. 148:2390–2394.49. Ying S, Durham SR, Barkans J, Masuyama K, Jacobson M, Rak S, Löwhagen O, Moqbel R, Kay AB, Hamid QA. T cells are the principal source of interleukin-5 mRNA in allergen-induced rhinitis. Am J Respir Cell Mol Biol. 1993. 9:356–360.
Article50. Varga EM, Jacobson MR, Till SJ, Masuyama K, O'Brien F, Rak S, Lund V, Scadding GK, Hamid QA, Durham SR. Cellular infiltration and cytokine mRNA expression in perennial allergic rhinitis. Allergy. 1999. 54:338–345.
Article51. Pawankar RU, Okuda M, Suzuki K, Okumura K, Ra C. Phenotypic and molecular characteristics of nasal mucosal gamma delta T cells in allergic and infectious rhinitis. Am J Respir Crit Care Med. 1996. 153:1655–1665.
Article52. Zuany-Amorim C, Ruffié C, Hailé S, Vargaftig BB, Pereira P, Pretolani M. Requirement for gammadelta T cells in allergic airway inflammation. Science. 1998. 280:1265–1267.53. Mészáros G, Szalay B, Toldi G, Mezei G, Tamási L, Vásárhelyi B, Cserhéti E, Treszl A. FoxP3+ regulatory T cells in childhood allergic rhinitis and asthma. J Investig Allergol Clin Immunol. 2009. 19:238–240.54. Provoost S, Maes T, van Durme YM, Gevaert P, Bachert C, Schmidt-Weber CB, Brusselle GG, Joos GF, Tournoy KG. Decreased FOXP3 protein expression in patients with asthma. Allergy. 2009. 64:1539–1546.
Article55. Juliusson S, Bachert C, Klementsson H, Karlsson G, Pipkorn U. Macrophages on the nasal mucosal surface in provoked and naturally occurring allergic rhinitis. Acta Otolaryngol. 1991. 111:946–953.
Article56. Fokkens WJ, Broekhuis-Fluitsma DM, Rijntjes E, Vroom TM, Hoefsmit EC. Langerhans cells in nasal mucosa of patients with grass pollen allergy. Immunobiology. 1991. 182:135–142.
Article57. Godthelp T, Fokkens WJ, Kleinjan A, Holm AF, Mulder PG, Prens EP, Rijntes E. Antigen presenting cells in the nasal mucosa of patients with allergic rhinitis during allergen provocation. Clin Exp Allergy. 1996. 26:677–688.
Article58. Fokkens WJ, Vroom TM, Rijntjes E, Mulder PG. CD-1 (T6), HLA-DR-expressing cells, presumably Langerhans cells, in nasal mucosa. Allergy. 1989. 44:167–172.
Article59. Shoji S, Ertl RF, Linder J, Koizumi S, Duckworth WC, Rennard SI. Bronchial epithelial cells respond to insulin and insulin-like growth factor-I as a chemoattractant. Am J Respir Cell Mol Biol. 1990. 2:553–557.
Article60. Campbell AM, Chanez P, Vignola AM, Bousquet J, Couret I, Michel FB, Godard P. Functional characteristics of bronchial epithelium obtained by brushing from asthmatic and normal subjects. Am Rev Respir Dis. 1993. 147:529–534.
Article61. Terada N, Maesako K, Hamano N, Houki G, Ikeda T, Sai M, Yamashita T, Fukuda S, Wakita A, Yoshimura K, Konno A. Eosinophil adhesion regulates RANTES production in nasal epithelial cells. J Immunol. 1997. 158:5464–5470.62. Lee BJ, Naclerio RM, Bochner BS, Taylor RM, Lim MC, Baroody FM. Nasal challenge with allergen upregulates the local expression of vascular endothelial adhesion molecules. J Allergy Clin Immunol. 1994. 94:1006–1016.
Article63. Pawankar R, Watanabe S, Nonaka M, Ozu C, Aida M, Yagi T. Differential expression of metalloproteinase 2 and 9 in the allergic nasal mucosa and nasal polyps. J Allergy Clin Immunol. 2004. 113:S332.64. Takizawa R, Pawankar R, Yamagishi S, Takenaka H, Yagi T. Increased expression of HLA-DR and CD86 in nasal epithelial cells in allergic rhinitics: antigen presentation to T cells and up-regulation by diesel exhaust particles. Clin Exp Allergy. 2007. 37:420–433.
Article65. Yamagishi S, Pawankar R, Takizawa R, Nonaka M, Yagi T. Nasal epithelial cells express the FcεRI: IL-4 induced upregulation of the FcεRI and IL-6 production. J Allergy Clin Immunol. 2003. 111:S347.
Article66. Roche N, Chinet TC, Huchon GJ. Allergic and nonallergic interactions between house dust mite allergens and airway mucosa. Eur Respir J. 1997. 10:719–726.67. Thompson PJ. Unique role of allergens and the epithelium in asthma. Clin Exp Allergy. 1998. 28:Suppl 5. 110–116.
Article68. King C, Brennan S, Thompson PJ, Stewart GA. Dust mite proteolytic allergens induce cytokine release from cultured airway epithelium. J Immunol. 1998. 161:3645–3651.69. Wan H, Winton HL, Soeller C, Tovey ER, Gruenert DC, Thompson PJ, Stewart GA, Taylor GW, Garrod DR, Cannell MB, Robinson C. Der p 1 facilitates transepithelial allergen delivery by disruption of tight junctions. J Clin Invest. 1999. 104:123–133.
Article70. Devalia JL, Bayram H, Abdelaziz MM, Sapsford RJ, Davies RJ. Differences between cytokine release from bronchial epithelial cells of asthmatic patients and non-asthmatic subjects: effect of exposure to diesel exhaust particles. Int Arch Allergy Immunol. 1999. 118:437–439.
Article71. Liu YJ. Thymic stromal lymphopoietin: master switch for allergic inflammation. J Exp Med. 2006. 203:269–273.
Article72. Rochman Y, Leonard WJ. Thymic stromal lymphopoietin: a new cytokine in asthma. Curr Opin Pharmacol. 2008. 8:249–254.
Article73. Miyata M, Hatsushika K, Ando T, Shimokawa N, Ohnuma Y, Katoh R, Suto H, Ogawa H, Masuyama K, Nakao A. Mast cell regulation of epithelial TSLP expression plays an important role in the development of allergic rhinitis. Eur J Immunol. 2008. 38:1487–1492.
Article74. Kimura S, Pawankar R, Mori S, Nonaka M, Masuno S, Yagi T, Okubo K. Increased expression and role of thymic stromal lymphopoietin in nasal polyposis. Allergy Asthma Immunol Res. 2011. 3:186–193.
Article75. Wang YH, Angkasekwinai P, Lu N, Voo KS, Arima K, Hanabuchi S, Hippe A, Corrigan CJ, Dong C, Homey B, Yao Z, Ying S, Huston DP, Liu YJ. IL-25 augments type 2 immune responses by enhancing the expansion and functions of TSLP-DC-activated Th2 memory cells. J Exp Med. 2007. 204:1837–1847.
Article76. Préfontaine D, Lajoie-Kadoch S, Foley S, Audusseau S, Olivenstein R, Halayko AJ, Lemière C, Martin JG, Hamid Q. Increased expression of IL-33 in severe asthma: evidence of expression by airway smooth muscle cells. J Immunol. 2009. 183:5094–5103.
Article77. Karlsson MG, Hellquist HB. Endothelial adhesion molecules for nasal-homing T cells in allergy. Virchows Arch. 1996. 429:49–54.
Article78. Terada N, Maesako K, Hamano N, Ikeda T, Sai M, Yamashita T, Fukuda S, Konno A. RANTES production in nasal epithelial cells and endothelial cells. J Allergy Clin Immunol. 1996. 98:S230–S237.
Article79. Jeannin P, Delneste Y, Gosset P, Molet S, Lassalle P, Hamid Q, Tsicopoulos A, Tonnel AB. Histamine induces interleukin-8 secretion by endothelial cells. Blood. 1994. 84:2229–2233.
Article80. Delneste Y, Lassalle P, Jeannin P, Joseph M, Tonnel AB, Gosset P. Histamine induces IL-6 production by human endothelial cells. Clin Exp Immunol. 1994. 98:344–349.
Article81. Malaviya R, Twesten NJ, Ross EA, Abraham SN, Pfeifer JD. Mast cells process bacterial Ags through a phagocytic route for class I MHC presentation to T cells. J Immunol. 1996. 156:1490–1496.82. Cameron L, Hamid Q, Wright E, Nakamura Y, Christodoulopoulos P, Muro S, Frenkiel S, Lavigne F, Durham S, Gould H. Local synthesis of epsilon germline gene transcripts, IL-4, and IL-13 in allergic nasal mucosa after ex vivo allergen exposure. J Allergy Clin Immunol. 2000. 106:46–52.83. Pawankar R, Yamagishi S, Yagi T. Revisiting the roles of mast cells in allergic rhinitis and its relation to local IgE synthesis. Am J Rhinol. 2000. 14:309–317.
Article84. Powe DG, Jagger C, Kleinjan A, Carney AS, Jenkins D, Jones NS. 'Entopy': localized mucosal allergic disease in the absence of systemic responses for atopy. Clin Exp Allergy. 2003. 33:1374–1379.
Article85. Nilsson G, Forsberg-Nilsson K, Xiang Z, Hallböök F, Nilsson K, Metcalfe DD. Human mast cells express functional TrkA and are a source of nerve growth factor. Eur J Immunol. 1997. 27:2295–2301.
Article86. Leon A, Buriani A, Dal Toso R, Fabris M, Romanello S, Aloe L, Levi-Montalcini R. Mast cells synthesize, store, and release nerve growth factor. Proc Natl Acad Sci U S A. 1994. 91:3739–3743.
Article87. Bienenstock J, Tomioka M, Matsuda H, Stead RH, Quinonez G, Simon GT, Coughlin MD, Denburg JA. The role of mast cells in inflammatory processes: evidence for nerve/mast cell interactions. Int Arch Allergy Appl Immunol. 1987. 82:238–243.
Article88. Virchow JC, Julius P, Lommatzsch M, Luttmann W, Renz H, Braun A. Neurotrophins are increased in bronchoalveolar lavage fluid after segmental allergen provocation. Am J Respir Crit Care Med. 1998. 158:2002–2005.
Article89. Ciprandi G, Ricca V, Tosca MA, Landi M, Passalacqua G, Canonica GW. Continuous antihistamine treatment controls allergic inflammation and reduces respiratory morbidity in children with mite allergy. Allergy. 1999. 54:358–365.
Article90. Braunstahl GJ, Overbeek SE, Kleinjan A, Prins JB, Hoogsteden HC, Fokkens WJ. Nasal allergen provocation induces adhesion molecule expression and tissue eosinophilia in upper and lower airways. J Allergy Clin Immunol. 2001. 107:469–476.
Article91. Corren J, Adinoff AD, Irvin CG. Changes in bronchial responsiveness following nasal provocation with allergen. J Allergy Clin Immunol. 1992. 89:611–618.
Article92. Crystal-Peters J, Neslusan C, Crown WH, Torres A. Treating allergic rhinitis in patients with comorbid asthma: the risk of asthma-related hospitalizations and emergency department visits. J Allergy Clin Immunol. 2002. 109:57–62.
Article93. Valovirta E, Pawankar R. Survey on the impact of comorbid allergic rhinitis in patients with asthma. BMC Pulm Med. 2006. 6:Suppl 1. S3.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diagnosis of Allergic Rhinitis
- Allergic Rhinitis Mouse Model
- Allergic Rhinitis and Sleep-disordered Breathing
- The Role of Aviation Medical Examiners in the Diagnosis, Treatment and Aeromedical Assessment of Patients with Allergic Rhinitis
- Dermographism ( IV ): The Prevalence in Atopic Dermatitis and Allergic Rhinitis